Volume 31, Number 9—September 2025
Dispatch
Imported Malaria and Congenital Acquisition in Infant, Portugal, 2024
Abstract
Plasmodium falciparum infection was diagnosed in a 3-month-old baby in Portugal by optical microscopy. The mother had had malaria in Angola 13 months earlier, before she emigrated to Portugal. She remained asymptomatic throughout and after pregnancy. We confirmed the diagnosis of an imported malaria case and congenital transmission using molecular techniques.
Imported malaria cases in Europe from sub-Saharan Africa countries can manifest with very low parasite densities and asymptomatic infections. Those infections can pose a public health threat, given the potential for onward transmission in areas with competent vectors and suitable conditions (1–3).
Congenital malaria in infants is rare because the placenta acts as an effective barrier preventing the transfer of malaria parasites from maternal to fetal circulatory system; transmission during labor is the most likely mechanism (4). Ultrasensitive molecular diagnostic tools detect infections in settings with low parasite density (5,6). Congenital malaria is not universally defined. Some authors define it as the presence of asexual forms of malaria parasites in an infant’s cord blood or peripheral blood during the first week of life, regardless of clinical symptoms (4,7). Others require the presence of parasites in the newborn’s peripheral blood on the first day of life for diagnosis (4,7). However, a timely diagnosis can be missed if a patient has no suggestive symptoms or clinical or travel history that prompt an early assessment. We report Plasmodium falciparum infection in a 3-month-old baby in Portugal.
A 3-month-old female infant was brought to a pediatric emergency department of Vila Franca de Xira Hospital (Vila Franca Xira, Portugal) with a 2-day history of fever and splenomegaly; maximum axillary temperature was 38.6°C. Initial laboratory tests revealed a hemoglobin level of 10.9 g/dL (reference threshold is 11.0 g/L for children 6–59 months of age), a leukocyte count of 10,500/mm3 (2,040/mm3 neutrophils and 5,970/mm3 lymphocytes) (reference ranges 7,300–16,600/mm3 for leukocytes, 1,500–6,900/mm3 for neutrophils, and 3,400–9,400/mm3 for lymphocytes), platelet count of 66,000/mm3 (reference range 180,000–440,000/mm3), and C-reactive protein level of 53.4 mg/L (reference range <10 mg/L). A peripheral blood smear revealed P. falciparum trophozoites; parasite count was estimated at 4.6%. Further investigation of family history revealed that the infant was born via cesarean delivery; infant and mother were discharged from hospital without any concerns. The infant had no history of traveling abroad, but her mother had moved to Portugal from Angola 13 months earlier. The mother had received artemether/lumefantrine treatment for P. falciparum infection in Angola 1 week before relocating to Portugal.
Because our findings were consistent with a suspected case of congenital malaria, we obtained samples from the mother and infant for molecular testing. We collected samples from the infant, with written consent from her mother, from the neonatal Guthrie card with blood collected via heel prick at the fourth day of life, during initial hospital admission, and at a follow-up appointment, at which we also took a sample from the mother. Maternal thick and thin blood smears did not reveal any malarial parasites; a rapid diagnostic test for malaria returned negative results.
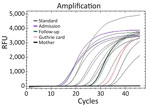
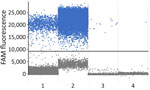
We performed total DNA extraction using a QIAamp DNA Mini Kit (QIAGEN, http://www.qiagen.com) on dried blood spots on Whatman filter paper. We tested samples by endpoint nested PCR, as described by Singh (8). We performed molecular assays (Table 1) to assess parasite density, using standard curves prepared with 10-fold dilutions of DNA obtained from the P. falciparum 3D7 clone; result range was 104 to 10−1 parasites/µL (Figure 1). We applied high-sensitivity quantitative PCR (qPCR) targeting the multicopy telomeric var genes to estimate the densities of the parasite. We performed digital PCR to confirm and compare results (Figure 2) (5).
This case highlights 2 key aspects for a better understanding of P. falciparum transmission: the role of imported, low-density asymptomatic cases as reservoirs and their potential contribution to congenital transmission. In Europe, ≈8,000 cases of imported malaria are reported annually (2); imported cases are mainly P. falciparum infections. Those populations can become a parasite reservoir that can pose significant risk to public health. Migrants may also display mild symptoms or be asymptomatic, often with submicroscopic parasitemia (2), which may be attributed to immunity acquired while residing in malaria-endemic regions (9). Such low levels of parasitemia can only be detected through sensitive molecular methods (10), such as qPCR-based techniques; we obtained the positive test result from this patient by nested PCR in the sample taken at hospital admission, when she was experiencing symptoms and parasitemia. In Portugal, there are potentially malaria-receptive areas and also a vector with some degree of competence (3). In non–malaria-endemic settings, availability of diagnostic tools varies by healthcare setting (Table 2). Nevertheless, clinicians should recognize the likelihood of congenital malaria and refer blood samples from suspected cases for testing.
var genes are known for their role in antigenic variation, enabling P. falciparum to evade the host immune response. The high copy number of var genes can enhance the sensitivity of detection methods. For instance, the use of multicopy subtelomeric targets has been shown to improve the detection of low-density infections that might otherwise be missed using standard assays such as 18ssRNA PCR (6). In our study, we established an accurate diagnosis for the mother through pfvarATS qPCR, which detected 0.03 parasites/µL, and digital PCR (dPCR), which detected 0.5 parasites/µL. Without the mother’s diagnosis, the child’s infection would have likely gone undetected and untreated. We have not established whether transmission to the infant was transplacental or from direct contact with maternal blood during labor. In most pregnancies resulting in congenital malaria, the mother’s infection tends to be symptomatic (11); malaria can also be diagnosed after uncomplicated asymptomatic pregnancies (11).
For our study, we defined congenital malaria as outlined in Oluput-Oluput (12) as the direct infection of an infant with malaria parasites from the mother before or during birth. Although all clinical indicators were consistent with congenital malaria, we pursued confirmation by detecting and quantifying parasite DNA in the Guthrie card sample taken on day 4 after birth. Both qPCR and dPCR confirmed the presence of P. falciparum parasites in the infant’s peripheral blood, with very low parasite densities (0.07 parasites/µL by qPCR and 0.49 parasites/µL by dPCR). Those findings align with the parasitemia levels observed in the mother, further supporting the likelihood of vertical transmission.
The delayed onset of symptoms in the infant can be attributed to several factors: the transfer of maternal antibodies through transplacental transfer and breastfeeding (13), low iron levels, and reduced erythropoiesis in newborns that do not favor Plasmodium spp. growth (14). Parasitemia might increase as maternal antibodies decline (15). In this case, the onset of symptoms occurred later than the previously reported median age, making the diagnosis more challenging (7). Furthermore, the nonspecific clinical signs of congenital malaria can be difficult to distinguish from other causes of sepsis (4). Our study highlights the importance of considering congenital malaria in the differential diagnosis of febrile infants born to mothers who have lived in malaria-endemic areas (7). We also emphasize the need for thorough analysis of blood smears in sepsis cases, particularly when thrombocytopenia is present (12).
In conclusion, this case underscores the utility of ultrasensitive detection targets and methods such as pfvartATS qPCR and dPCR for detecting submicroscopic malaria infections, particularly in asymptomatic migrant populations or populations at higher risk such as pregnant women and infants. Our findings emphasize the public health risk of overlooking hidden parasite reservoirs, which could hinder effective malaria control and prevention efforts in vulnerable groups.
Dr. Lopes is a senior technician at the Global Health and Tropical Medicine Research Centre, Instituto de Higiene e Medicina Tropical, NOVA University Lisbon. Her primary research and teaching interests are malaria and other neglected tropical diseases, focusing on development and capacity-building in tropical disease diagnostics and public health, mainly in Portuguese-speaking countries in Africa.
Acknowledgments
We thank the patient’s mother for consenting to the publication of these results. We thank Rui Batista, Gonçalo Carvalho, and Sara Mateus for their invaluable technical support during the digital PCR experiments and analysis workflow.
Fundação para a Ciência e a Tecnologia supported this work (GHTM—UID/04413/2020 and LA-REAL–LA/P/0117/2020).
References
- Corbacho-Loarte MD, Crespillo-Andújar C, Chamorro-Tojeiro S, Norman F, Pérez-Molina JA, Martín O, et al. Screening of imported malaria infection in asymptomatic migrants from Sub-Saharan Africa: A retrospective analysis of a 2010-2019 cohort. Travel Med Infect Dis. 2022;49:
102411 . DOIPubMedGoogle Scholar - Pousibet-Puerto J, Lozano-Serrano AB, Soriano-Pérez MJ, Vázquez-Villegas J, Giménez-López MJ, Cabeza-Barrera MI, et al. Migration-associated malaria from Africa in southern Spain. Parasit Vectors. 2021;14:240. DOIPubMedGoogle Scholar
- Gomes E, Capinha C, Rocha J, Sousa C. Mapping risk of malaria transmission in mainland Portugal using a mathematical modelling approach. PLoS One. 2016;11:
e0164788 . DOIPubMedGoogle Scholar - Bilal JA, Malik EE, Al-Nafeesah A, Adam I. Global prevalence of congenital malaria: A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;252:534–42. DOIPubMedGoogle Scholar
- Dong L, Li W, Xu Q, Gu J, Kang Z, Chen J, et al. A rapid multiplex assay of human malaria parasites by digital PCR. Clin Chim Acta. 2023;539:70–8. DOIPubMedGoogle Scholar
- Hofmann N, Mwingira F, Shekalaghe S, Robinson LJ, Mueller I, Felger I. Ultra-sensitive detection of Plasmodium falciparum by amplification of multi-copy subtelomeric targets. PLoS Med. 2015;12:
e1001788 . DOIPubMedGoogle Scholar - Prior AR, Prata F, Mouzinho A, Marques JG. Congenital malaria in a European country. BMJ Case Rep. 2012;2012:bcr2012007310. DOIGoogle Scholar
- Singh B, Bobogare A, Cox-Singh J, Snounou G, Abdullah MS, Rahman HA. A genus- and species-specific nested polymerase chain reaction malaria detection assay for epidemiologic studies. Am J Trop Med Hyg. 1999;60:687–92. DOIPubMedGoogle Scholar
- Cirera L, Sacoor C, Meremikwu M, Ranaivo L, F Manun’Ebo M, Arikpo D, et al. The economic costs of malaria in pregnancy: evidence from four sub-Saharan countries. Gates Open Res. 2023;7:47. DOIPubMedGoogle Scholar
- Omer S, Khalil E, Ali H, Sharief A. Submicroscopic and multiple plasmodium falciparum infections in pregnant Sudanese women. N Am J Med Sci. 2011;3:137–41. DOIPubMedGoogle Scholar
- Morven S. Edwards. Fungal and protozoal infections. In: Fanaroff AA, Martin RJ, editors. Neonatal-perinatal medicine: diseases of the fetus and infant. 7th ed. St. Louis: Mosby; 2002. p. 751–752.
- Olupot-Olupot P, Eregu EIE, Naizuli K, Ikiror J, Acom L, Burgoine K. Neonatal and congenital malaria: a case series in malaria endemic eastern Uganda. Malar J. 2018;17:171. DOIPubMedGoogle Scholar
- Natama HM, Moncunill G, Vidal M, Rouamba T, Aguilar R, Santano R, et al. Associations between prenatal malaria exposure, maternal antibodies at birth, and malaria susceptibility during the first year of life in Burkina Faso. Infect Immun. 2023;91:
e0026823 . DOIPubMedGoogle Scholar - Stassijns J, van den Boogaard W, Pannus P, Nkunzimana A, Rosanas-Urgell A. Prevalence and diagnostics of congenital malaria in rural Burundi, a cross-sectional study. Malar J. 2016;15:443–6. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: August 20, 2025
1These authors contributed equally to this article.
Table of Contents – Volume 31, Number 9—September 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Dinora Lopes, Universidade Nova de Lisboa, Instituto de Higiene e Medicina Tropical, UEI Tropical Clinic, Science and Community Support Service (SACC), Rua da Junqueira, 100 Lisbon, Lisbon 1349 008 Portugal
Top