Volume 6, Number 1—February 2000
Dispatch
Integronlike Structures in Campylobacter spp. of Human and Animal Origin
Figure 1
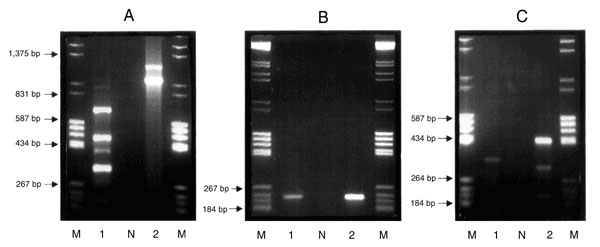
Figure 1. Agarose (1.5%) gel electrophoresis in 1x TAE buffer containing 0.5 µg/ml ethidium bromide. Purified genomic DNA was used as the template in a PCR reaction with the primers Int 1 F 5'-GGC ATC CAA GCA CGA AG-3' and Int 1 B 5'-AAG CAG ACT TGA CCT GA-3' (12). Lane M contains an equal mixture of molecular weight markers grades III and V (Boehringer Mannheim, Germany), Lane N is the negative containing all reaction components with the exception of template DNA, Lane 1, Campylobacter coli CIT-H 6 (IP-I); Lane 2, Salmonella Typhimurium DT104 CIT-F 100 (IP-C). As in A above except that the primers used were qacEΔ1 F 5'-AT GCA ATA GTT GGC GAA GT-3'and qacEΔ1 B 5'-CAA GCT TTT GCC CAT GAA GC-3' (13). As in A above using primers sul1 F 5'-CTT CGA TGA GAG CCG GCG GC-3' and sul1 B 5'-GCA AGG CGG AAA CCC GCG CC-3' (13).
References
- Stern NJ. Reservoirs for Camplobacter jejuni and approaches for intervention in poultry. In: Nachamkin I, Blaser MJ, Tompkins LS, editors. Campylobacter jejuni: current status and future trends. Washington: American Society for Microbiology; 1992. p. 49-60.
- Skirrow MB. Diseases due to Campylobacter, Helicobacter and related bacteria. J Comp Pathol. 1994;111:113–49. DOIPubMedGoogle Scholar
- Nachamkin I, Allos BM, Ho T. Campylobacter species and Guillain-Barré syndrome. Clin Microbiol Rev. 1998;11:555–67.PubMedGoogle Scholar
- Recchia GD, Hall RM. Gene cassettes: a new class of mobile element. Microbiology. 1995;141:3015–27. DOIPubMedGoogle Scholar
- Hall RM, Collis CM. Mobile gene cassettes and integrons: capture and spread of genes by site-specific recombination. Mol Microbiol. 1995;15:593–600. DOIPubMedGoogle Scholar
- Moore JE, Elisha BG. Workshop summary: session D. Campylobacter and Helicobacter: antibiotic resistance. In: Lastovica AJ, Newell DG, Lastovica EE, editors. Campylobacter, Helicobacter & related organisms, Institute of Child Health, University of Cape Town; 1998. p. 133-5.
- Ruiz J, GoZi P, Marco F, Gallardo F, Mirelis B, De Anta TJ, . Increased resistance to quinolones in Campylobacter jejuni: a genetic analysis of gyrA gene mutations in quinolone-resistant clinical isolates. Microbiol Immunol. 1998;42:223–6.PubMedGoogle Scholar
- Sjögren E, Kaijser B, Werner M. Antimicrobial susceptibilities of Campylobacter jejuni and Campylobacter coli isolated in Sweden: a 10-year follow-up report. Antimicrob Agents Chemother. 1992;36:2847–9.PubMedGoogle Scholar
- Reina J, Ros MJ, Serra A. Susceptibilities to ten antimicrobial agents of 1,120 Campylobacter strains isolated from 1987 to 1993 from faeces of paediatric patients. Antimicrob Agents Chemother. 1994;39:2910–20.
- National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disc susceptibility tests. Vol. 1, p: 141-56 (1981). Approved standard. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- Mazurier S, Van de Giessen A, Heuvelman K, Wernars K. RAPD analysis of Campylobacter isolates: DNA fingerprinting without the need to purify DNA. Lett Appl Microbiol. 1992;14:260–2. DOIPubMedGoogle Scholar
- Levesque C, Pichè L, Larose C, Roy P. PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob Agents Chemother. 1995;39:185–91.PubMedGoogle Scholar
- Sandvang D, Aarestrup FM, Jensen LB. Characterisation of integrons and antibiotic resistance genes in Danish multiresistant Salmonella enterica typhimurium DT104. FEMS Microbiol Lett. 1998;160:37–41. DOIPubMedGoogle Scholar
- Daly M, Buckley J, Power E, O'Hare C, Cormican M, Cryan B, Molecular characterization of Irish Salmonella enterica serotype Typhimurium: detection of class 1 integrons and assessment of genetic relationships by DNA fingerprinting. Appl Environ Microbiol. 2000. In press.
- Gibreel A, Sköld O. High-level resistance to trimethoprim in clinical isolates of Campylobacter jejuni by acquisition of foreign genes (dfr1 and dfr9) expressing drug-insensitive dihydrofolate reductases. Antimicrob Agents Chemother. 1998;42:3059–64.PubMedGoogle Scholar
- Briggs C, Fratamico PM. Molecular characterization of an antibiotic resistance gene cluster of Salmonella typhimurium DT104. Antimicrob Agents Chemother. 1999;43:846–9. DOIPubMedGoogle Scholar
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–402. DOIPubMedGoogle Scholar
- Wang Y, Taylor DE. Natural transformation in Campylobacter species. J Bacteriol. 1990;172:949–55.PubMedGoogle Scholar
- Jackson CJ, Fox AJ, Jones DM, Wareing DRA, Hutchinson DN. Associations between heat-stable (O) and heat-labile (HL) serogroup antigens of Campylobacter jejuni: evidence for interstrain relationships within three O/HL serovars. J Clin Microbiol. 1998;36:2223–8.PubMedGoogle Scholar
- On SLW, Nielsen EM, Engberg J, Madsen M. Validity of SmaI-defined genotypes of Campylobacter jejuni examined by SalI, KpnI, and BamHI polymorphisms: evidence of identical clones infecting humans, poultry, and cattle. Epidemiol Infect. 1998;120:231–7. DOIPubMedGoogle Scholar
- Richardson PT, Park SF. Integration of heterologous plasmid DNA into multiple sites on the genome of Campylobacter coli following natural transformation. J Bacteriol. 1997;179:1809–12.PubMedGoogle Scholar
- Kokotovic B, On SLW. High-resolution genomic fingerprinting of Campylobacter jejuni and Campylobacter coli by analysis of amplified fragment length polymorphisms. FEMS Microbiol Lett. 1999;173:77–84. DOIPubMedGoogle Scholar
- Sallen B, Rajoharison A, Desvarenne S, Mabilat C. Molecular epidemiology of integron-associated antibiotic resistance genes in clinical isolates of Enterobacteriaceae. Microb Drug Resist. 1995;1:195–202. DOIPubMedGoogle Scholar