Volume 7, Number 2—April 2001
THEME ISSUE
4th Decennial International Conference on Nosocomial and Healthcare-Associated Infections
State of the Art
Engineering out the Risk of Infection with Urinary Catheters
Definition of CAUTI
Risk Factors for CAUTI
Guidelines for Preventing CAUTI
Avoid Unnecessary Catheterizations
Consider Alternatives to Urethral Catheterization
Insertion Using Aseptic Technique
Closed Drainage
Ensure Dependent Drainage
Urine Collection
Other Practices
Novel Technology
The Future
Cite This Article
Cite This Article
Citation for Media
Abstract
Catheter-associated urinary tract infection (CAUTI) is the most common nosocomial infection. Each year, more than 1 million patients in U.S. acute-care hospitals and extended-care facilities acquire such an infection; the risk with short-term catheterization is 5% per day. CAUTI is the second most common cause of nosocomial bloodstream infection, and studies suggest that patients with CAUTI have an increased institutional death rate, unrelated to the development of urosepsis. Novel urinary catheters impregnated with nitrofurazone or minocycline and rifampin or coated with a silver alloy-hydrogel exhibit antiinfective surface activity that significantly reduces the risk of CAUTI for short-term catheterizations not exceeding 2-3 weeks.
Each year, urinary catheters are inserted in more than 5 million patients in acute-care hospitals and extended-care facilities. Catheter-associated urinary tract infection (CAUTI) is the most common nosocomial infection in hospitals and nursing homes, comprising >40% of all institutionally acquired infections (1–4). Nosocomial bacteriuria or candiduria develops in up to 25% of patients requiring a urinary catheter for >7 days, with a daily risk of 5% (5–7). CAUTI is the second most common cause of nosocomial bloodstream infection (8–10), and studies by Platt et al. (11) and Kunin et al. (12) suggest that nosocomial CAUTIs are associated with substantially increased institutional death rates, unrelated to the occurrence of urosepsis. Although most CAUTIs are asymptomatic (13), rarely extend hospitalization, and add only $500 to $1,000 to the direct costs of acute-care hospitalization (14), asymptomatic infections commonly precipitate unnecessary antimicrobial-drug therapy. CAUTIs comprise perhaps the largest institutional reservoir of nosocomial antibiotic-resistant pathogens (5–10,15), the most important of which are multidrug-resistant Enterobacteriacae other than Escherichia coli, such as Klebsiella, Enterobacter, Proteus, and Citrobacter; Pseudomonas aeruginosa; enterococci and staphylococci; and Candida spp. (Table 1).
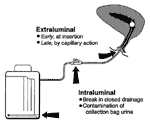
Excluding rare hematogenously derived pyelonephritis, caused almost exclusively by Staphylococcus aureus, most microorganisms causing endemic CAUTI derive from the patient's own colonic and perineal flora or from the hands of health-care personnel during catheter insertion or manipulation of the collection system. Organisms gain access in one of two ways (Figure 1). Extraluminal contamination may occur early, by direct inoculation when the catheter is inserted, or later, by organisms ascending from the perineum by capillary action in the thin mucous film contiguous to the external catheter surface. Intraluminal contamination occurs by reflux of microorganisms gaining access to the catheter lumen from failure of closed drainage or contamination of urine in the collection bag.
Recent studies suggest that CAUTIs most frequently stem from microorganisms gaining access to the bladder extraluminally, but both routes are important (Table 2) (16). Some studies suggest that the extraluminal route may be of greater relative importance in women because of the short urethra and its close proximity to the anus (17). Investigators have found that antecedent heavy periurethral cutaneous colonization is an important risk factor for CAUTI in both men and women (17,18).
Most infected urinary catheters are covered by a thick biofilm containing the infecting microorganisms embedded in a matrix of host proteins and microbial exoglycocalyx (Figure 2). A biofilm forms intraluminally, extraluminally, or both ways, usually advancing in a retrograde fashion (19). The role of the biofilm in the pathogenesis of CAUTI has not been established. However, antiinfective-impregnated and silver-hydrogel catheters (20–26), which inhibit adherence of microorganisms to the catheter surface, significantly reduce the risk of CAUTI, particularly infections caused by gram-positive organisms or yeasts, which are most likely to be acquired extraluminally from the periurethral flora (16). These data suggest that microbial adherence to the catheter surface is important in the pathogenesis of many, but not all, CAUTIs. Infections in which the biofilm does not play a pathogenetic role are probably caused by mass transport of intraluminal contaminants into the bladder by retrograde reflux of microbe-laden urine when a catheter or collection system is moved or manipulated (Figure 1, Table 2).
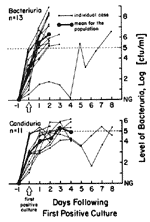
A prospective study in which catheterized patients were cultured daily by a technique capable of detecting very low- level bacteriuria, as low as 1 CFU/mL (7), showed that isolation of any microorganisms from an intraluminal specimen, even 3-4 CFU/mL, is highly predictive of CAUTI. If intercurrent antimicrobial therapy is not given, the level of bacteriuria or candiduria almost uniformly increases to >105 within 24-48 hours (Figure 3), demonstrating the vulnerability of the catheterized urinary tract to infection once any microorganisms gain access to the lumen of the catheter and the bladder. The very heavy use of systemic antimicrobial drugs in catheterized patients, which has been found in most studies (5–13), probably keeps the rate of CAUTI considerably lower than it would be otherwise, but unfortunately selects for the resistant organisms that produce most nosocomial CAUTIs (Table 1).
Most clinicians use a clean-voided specimen showing >105 CFU/mL as the criterion for "significant" bacteriuria (i.e., true infection) for noncatheterized patients (4). However, once any microorganisms are identified in urine from a patient's indwelling catheter, unless suppressive antimicrobial-drug therapy is being given or started, progression to concentrations >105 CFU/mL occurs predictably and rapidly, usually within 72 hours (Figure 3) (7). Thus, most authorities consider concentrations >102 or 103 CFU/mL, in urine collected with a needle from the sampling port of the catheter, to be indicative of true CAUTI. This concentration can be reproducibly detected in the laboratory, and this definition is useful for therapeutic decisions and epidemiologic research (1–7).
Large, prospective studies in which catheterized patients were cultured daily and which used multivariable techniques of statistical analysis identified risk factors independently predictive of increased risk for CAUTI (27-30; Table 3). Females have a substantially higher risk than males (relative risk [RR] 2.5-3.7), and patients with other active sites of infection (RR 2.3 - 2.4) or a major preexisting chronic condition (such as diabetes [RR 2.2-2.3], malnutrition [RR 2.4], or renal insufficiency [RR 2.1-2.6]) also are at higher risk. Inserting the catheter outside the operating room (RR 2.0-5.3) or late in hospitalization (RR 2.6-8.6), presence of a ureteral stent (RR 2.5), or using the catheter to measure urine output (RR 2.0) further increase the risk.
The most important, potentially modifiable risk factor, identified in every study, is prolonged catheterization, beyond 6 days (RR 5.1-6.8); by the 30th day of catheterization, infection is near-universal. A large, prospective study monitored compliance on a daily basis with seven recommended precepts for catheter care, including closed drainage, dependent drainage including proper position of the drainage tubing and collection bag, and protection of the drainage port; the only violation predictive of an increased risk of CAUTI was improper position of the drainage tube, above the level of the bladder or sagging below the level of the collection bag (RR 1.9) (27).
Antimicrobial-drug therapy has been shown to be protective against CAUTI for short-term catheterizations (RR 0.001-0.4) but clearly selects for infection caused by multidrug-resistant microorganisms, such as P. aeruginosa, and other resistant gram-negative bacilli, enterococci, and yeasts (Table 1) (1–10,15).
Several catheter-care practices are universally recommended to prevent or at least delay the onset of CAUTI: avoid unnecessary catheterizations; consider a condom or suprapubic catheter; have a trained professional insert the catheter aseptically; remove the catheter as soon as no longer needed; maintain uncompromising closed drainage; ensure dependent drainage; minimize manipulations of the system; and separate catherized patients (1–4). However, few of these practices have been proven to be effective by randomized controlled trials.
Use of indwelling urethral catheters should be limited to patients requiring relief of anatomic or physiologic outlet obstruction; patients undergoing surgical repair of the genitourinary tract (to facilitate healing); critically ill or postoperative patients who need their urinary output accurately measured; and debilitated, paralyzed, or comatose patients (to prevent skin breakdown and infected pressure ulcers). When no longer needed, the catheter should be promptly removed (31).
Suprapubic catheterization is more comfortable and acceptable to the patient and may be associated with a lower incidence of CAUTI (32). For incontinent males who do not have bladder outlet obstruction, condom drainage, while not free from nosocomial urinary tract infections, appears to be associated with a lower risk than indwelling urethral catheters (33).
Catheters should be inserted by trained health-care professionals using aseptic technique, including sterile gloves, a fenestrated sterile drape, and an effective cutaneous antiseptic, such as 10% povidone-iodine or 1% to 2% aqueous chlorhexidine.
After a catheter is inserted, uncompromising maintenance of closed drainage is of the highest priority and can keep the overall risk of CAUTI <25% for up to 2 weeks of catheterization (5,6).
The collection tubing and bag should always remain below the level of the patient's bladder, but the drainage tubing should always be above the level of the collection bag. In one large prospective study, this was the only catheter-care violation associated with a significantly increased risk of CAUTI (RR 1.9) (27).
The catheter and the drainage system should be manipulated as little as possible, and urine output should be monitored hourly only when clearly indicated by the patient's condition.
If feasible, separating catheterized patients geographically on a patient-care unit may reduce the risk of cross-infection with multidrug-resistant nosocomial organisms such as Serratia, Klebsiella, Pseudomonas, and Enterobacter (34).
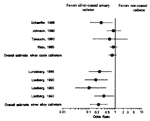
Systemic antimicrobial prophylaxis with trimethoprim-sulfamethoxazole, methenamine mandelate or, especially, a fluoroquinolone, can reduce the risk of CAUTI for short-term catheterizations (35). Although use of antimicrobials in this way may reduce the rate of CAUTI, infections that do occur are far more likely to be caused by antibiotic-resistant bacteria and yeasts (1–10). Since most CAUTIs are asymptomatic and do not result in urosepsis (13), it is difficult to justify antimicrobial therapy of asymptomatic bacteriuria other than for granulocytopenic or other severely immunocompromised patients, patients scheduled for urologic surgery, pregnant women, patients with Serratia CAUTI, or patients about to have their catheter removed. The societal benefits of antibiotic prophylaxis in immunocompetent catheterized patients to prevent largely asymptomatic CAUTIs are dubious.
Technologic innovations to prevent nosocomial infection are most likely to be most effective if they are based on a clear understanding of the pathogenesis and epidemiology of the infection (36). Novel technologies must be designed to block CAUTI by either the extraluminal or intraluminal routes or both (Figure 1). Technologic innovations have been proposed and evaluated during the past 25 years but have not proven conclusively beneficial (1–5). Among these innovations are using antiinfective lubricants when inserting the catheter; soaking the catheter in an antiinfective antimicrobial-drug solution before insertion; regular metal cleansing or periodically applying antiinfective creams or ointments to metals; continuously irrigating the catheterized bladder with an antiinfective solution through a triple-lumen catheter; or periodically instilling an antiinfective solution into the collection bag (Table 4). Bladder irrigation with antimicrobial-drug solutions has not only shown no benefit for prevention but has been associated with a strikingly increased proportion of CAUTIs caused by microorganisms resistant to the drugs in the irrigating solution (37).
Given the widely accepted importance of closed catheter drainage, efforts have been made to seal the connection between the catheter and collection tubing. An initial trial with a novel catheter showed a modest benefit and suggested a reduction in hospital deaths (38); however, follow-up studies have not demonstrated a reduction in CAUTI with a sealed catheter-collecting tube junction (39,40).
Medicated catheters, which reduce adherence of microorganisms to the catheter surface, may confer the greatest benefit for preventing CAUTI. Two catheters impregnated with antiinfective solutions have been studied in randomized trials, one impregnated with the urinary antiseptic nitrofurazone (20) and the other with a new broad-spectrum antimicrobial-drug combination, minocycline and rifampin (21). Both catheters showed a significant reduction in bacterial CAUTIs; however, the studies were small, and selection of antimicrobial-drug resistant uropathogens was not satisfactorily resolved.
The universal presence of a biofilm on the surface of an infected catheter (19) (Figure 2) has prompted hope that coating the catheter surface with an antiseptic, such as a silver compound, might reduce the risk for CAUTI. However, silver oxide-coated catheters, which had been initially reported to show promise, did not show efficacy when studied in large, well-controlled trials (29,30). In one of the trials, male patients with the coated catheter who did not receive systemic antibiotics had a paradoxical and inexplicably increased risk for CAUTI (30).
A silver-hydrogel catheter has been developed that inhibits adherence of microorganisms to the catheter surface in vitro; tested microorganisms include resistant enterococci, staphylococci, Enterobacteriaceae, P. aeruginosa, and yeasts (41). Small comparative but nonblinded trials have shown this product prevents CAUTI (22–25,42) (Figure 4). In a recent, large, double-blinded trial in 850 patients (26), the silver-hydrogel catheter reduced the incidence of CAUTI 26% (25.7 vs. 15.4 per 100 catheters, RR 0.74, p =0.04) (27). The greatest benefit was preventing infections caused by gram-positive organisms, enterococci and staphylococci (RR 0.45, p <0.001), and Candida (RR 0.80), microorganisms that usually gain access to the bladder extraluminally (16). The catheter conferred no protection against CAUTIs with gram-negative bacilli, which most often gain access intraluminally (16). Use of the silver-hydrogel catheter was not associated with an increased incidence of infections caused by antibiotic-resistant bacteria or Candida, and in vitro susceptibility testing of isolates from both treatment groups showed no infections caused by silver-resistant microorganisms. Cost-utility analysis indicates that use of this catheter could bring substantial cost savings to health-care institutions (Table 5).
The first major advance for preventing CAUTI since the wide-scale adoption of closed drainage 35 years ago is the development of catheters with antiinfective surfaces. These advances should not be considered the final answer, however. Other technologies that should be pursued include new, more potent antiinfective materials; microbe-impervious antireflux valves; urethral stents; conformable (collapsible) urethral catheters; and vaccines for enteric gram-negative bacilli and staphylococci. Antiseptics are far more likely than antibacterials to confer greater resistance to surface colonization and not to select for infection with antimicrobial-drug resistant bacteria or yeasts (43). New surface technologies that release far greater quantities of ionic silver or other antiinfective agents into the aqueous environment contiguous to the catheter surface might even prevent CAUTIs caused by intraluminal contaminants.
In uncontrolled trials, urethral stents have provided a less-invasive alternative to catheter drainage for men with outlet obstruction caused by prostatic hypertrophy or cancer (44). A conformable catheter, with a collapsible intraurethral segment that may cause less trauma to the urethra, has been developed but has not been tested clinically and is not commercially available. These and other alternatives to the rigid urethral catheter, such as a condom catheter for female patients (45), need to be evaluated in controlled, randomized trials.
The greatest hope for a major reduction in CAUTI and indeed all nosocomial infections is likely to be vaccines against important nosocomial multidrug-resistant pathogens, such as the enteric gram-negative bacilli and staphylococci.
Dr. Maki is professor of medicine and head of the Section of Infectious Diseases at the University of Wisconsin Medical School and hospital epidemiologist at University of Wisconsin Hospitals and Clinics. He has had a long interest in the pathogenesis, epidemiology, and prevention of nosocomial infections, particularly those caused by catheters or other implanted medical devices.
Dr. Tambyah, formerly a clinical and research fellow in infectious diseases at the University of Wisconsin Medical School, is assistant professor of medicine and consultant infectious disease physician at the University of Singapore School of Medicine and hospital epidemiologist at the National University Hospital of Singapore.
References
- Stamm WE. Catheter-associated urinary tract infections: Epidemiology, pathogenesis, and prevention. Am J Med. 1991;91(Suppl 3B):65S–71S. DOIPubMedGoogle Scholar
- Burke JP, Riley DK. Nosocomial urinary tract infection. In: Mayhall CG, editor. Hospital epidemiology and infection control. Baltimore: Williams and Wilkins; 1996. p. 139-53.
- Warren JW. Catheter-associated urinary tract infections. Infect Dis Clin North Am. 1997;11:609–22. DOIPubMedGoogle Scholar
- Kunin CM. Care of the urinary catheter. In: Urinary tract infections: detection, prevention and management. Fifth ed. Baltimore: Williams and Wilkins; 1997. p. 227-99.
- Kunin CM, McCormack RC. Prevention of catheter-induced urinary-tract infections by sterile closed drainage. N Engl J Med. 1966;274:1155–61. DOIPubMedGoogle Scholar
- Garibaldi RA, Mooney BR, Epstein BJ, Britt MR. An evaluation of daily bacteriologic monitoring to identify preventable episodes of catheter associated UTI. Infect Control. 1982;3:466–70.PubMedGoogle Scholar
- Stark RP, Maki DG. Bacteriuria in the catheterized patient. N Engl J Med. 1984;311:560–4. DOIPubMedGoogle Scholar
- Maki DG. Nosocomial bacteremia. An epidemiologic overview. Am J Med. 1981;70:719–32. DOIPubMedGoogle Scholar
- Krieger JN, Kaiser DIL, Wenzel RP. Urinary tract etiology of bloodstream infections in hospitalized patients. J Infect Dis. 1983;148:57–62. DOIPubMedGoogle Scholar
- Bryan CS, Reynolds KL. Hospital-acquired bacteremic urinary tract infection: epidemiology and outcome. J Urol. 1984;132:494–8.PubMedGoogle Scholar
- Platt R, Polk BF, Murdock B, Rosner B. Mortality associated with nosocomial urinary-tract infection. N Engl J Med. 1982;307:637–41. DOIPubMedGoogle Scholar
- Kunin CM, Douthitt S, Dancing J, Anderson J, Moeschberger M. The association between the use of urinary catheters and morbidity and mortality among elderly patients in nursing homes. Am J Epidemiol. 1992;135:291–301.PubMedGoogle Scholar
- Tambyah PA, Maki DG. Catheter-associated urinary tract infection is rarely symptomatic: a prospective study of 1497 catheterized patients. Arch Intern Med. 2000;160:678–82. DOIPubMedGoogle Scholar
- Patton JP, Nash DB, Abrutyn E. Urinary tract infection: economic considerations. Med Clin North Am. 1991;75:495–513.PubMedGoogle Scholar
- Jarvis WR, Martone WJ. Predominant pathogens in hospital infections. J Antimicrob Chemother. 1992;29:19–24.PubMedGoogle Scholar
- Tambyah PA, Halvorson K, Maki DG. A prospective study of the pathogenesis of catheter-associated urinary tract infection. Mayo Clin Proc. 1999;74:131–6. DOIPubMedGoogle Scholar
- Daifuku R, Stamm WE. Association of rectal and urethral colonization with urinary tract infection in patients with indwelling catheters. JAMA. 1984;252:2028–30. DOIPubMedGoogle Scholar
- Garibaldi RA, Burke JP, Britt MR, Miller MA, Smith CB. Metal colonization and catheter-associated bacteriuria. N Engl J Med. 1980;303:316–8. DOIPubMedGoogle Scholar
- Nickel JC, Costerton JW, McLean RJ, Olson M. Bacterial biofilms: influence on the pathogenesis, diagnosis and treatment of urinary tract infections. J Antimicrob Chemother. 1994;33(Suppl A):31–41.PubMedGoogle Scholar
- Maki DG, Knasinski V, Halvorson KT, Tambyah PA, Holcomb RG. A prospective, randomized, investigator-blinded trial of a novel nitrofurazone-impregnated urinary catheter [abstract M49]. Infect Control Hosp Epidemiol. 1997;18(Suppl):50.
- Darouiche RO, Smith A, Hanna H, Dhabuwala CB, Steiner MS, Babaian RJ, Efficacy of antimicrobial-impregnated bladder catheters in reducing catheter-associated bacteriuria: a prospective, randomized multicenter clinical trial. Urology. 1999;54:976–81. DOIPubMedGoogle Scholar
- Lundeberg T. Prevention of catheter-associated urinary tract infections by use of silver-impregnated catheters [letter]. Lancet. 1986;1:1031. DOIPubMedGoogle Scholar
- Liedberg H, Lundeberg T. Silver alloy coated catheters reduce catheter-associated bacteriuria. Br J Urol. 1990;65:379–81. DOIPubMedGoogle Scholar
- Liedberg H, Lundeberg T, Ekman P. Refinements in the coating of urethral catheters reduce the incidence of catheter-associated bacteriuria. An experimental and clinical study. Eur Urol. 1990;17:236–40.PubMedGoogle Scholar
- Liedberg H, Lundeberg T. Prospective study of incidence of urinary tract infection in patients catheterized with bard hydrogel and silver-coated catheters or bard hydrogel-coated catheters [abstract 405A]. J Urol. 1993; 149.PubMedGoogle Scholar
- Maki DG, Knasinski V, Halvorson K, Tambyah PA. A novel silver-hydrogelimpregnated indwelling catheter reduces CAUTIs: a prospective double-blind trial [abstract]. In: Programs and abstracts of the Society for Healthcare Epidemiology in America Annual Meeting; April 5-7, 1998; Orlando, Florida.
- Maki DG, Knasinski V, Tambyah PA. Risk factors for catheter-associated urinary tract infection: a prospective study showing the minimal effects of catheter care violations on the risk of CAUTI [abstract]. Infect Control Hosp Epidemiol. 2000;21:165.
- Platt R, Polk BF, Murdock B, Rosner B. Risk factors for nosocomial urinary tract infection. Am J Epidemiol. 1986;124:977–85.PubMedGoogle Scholar
- Johnson JR, Roberts PL, Olsen RJ, Moyer KA, Stamm WE. Prevention of catheter-associated urinary tract infection with a silver oxide-coated urinary catheter: Clinical and microbiologic correlates. J Infect Dis. 1990;162:1145–50. DOIPubMedGoogle Scholar
- Riley DK, Classen DC, Stevens LE, Burke JP. A large randomized clinical trial of a silver-impregnated urinary catheter: Lack of efficacy and staphylococcal superinfection. Am J Med. 1995;98:349–56. DOIPubMedGoogle Scholar
- Rabkin DG, Stifelman MD, Birkhoff J, Richardson KA, Cohen D, Nowygrod R, Early catheter removal decreases incidence of urinary tract infections in renal transplant recipients. Transplant Proc. 1998;30:4314–6. DOIPubMedGoogle Scholar
- Shapiro J, Hoffmann J, Jersky J. A comparison of suprapubic and transurethral drainage for postoperative urinary retention in general surgical patients. Acta Chir Scand. 1982;148:323–7.PubMedGoogle Scholar
- Warren JW. Urethral catheters, condom catheters, and nosocomial urinary tract infections. Infect Control Hosp Epidemiol. 1996;17:212–4. DOIPubMedGoogle Scholar
- Maki DG, Hennekens C, Bennet J. Prevention of catheter-associated urinary ract infection. JAMA. 1972;221:1270–1. DOIPubMedGoogle Scholar
- van der Wall E, Verkooyen RP, Mintjes-de-Groot J, Oostinga J, Van Dijk A, Hustius WN, Prophylactic ciprofloxacin for catheter-associated urinary tract infection. Lancet. 1992;339:946–51. DOIPubMedGoogle Scholar
- Maki DG. Risk factors for nosocomial infection in intensive care. "Devices vs nature" and goals for the next decade. Arch Intern Med. 1989;149:30–5. DOIPubMedGoogle Scholar
- Warren JW, Platt R, Thomas RJ, Rosner B, Kass EH. Antibiotic irrigation and catheter-associated urinary tract infections. N Engl J Med. 1978;299:570–3. DOIPubMedGoogle Scholar
- Platt R, Polk BF, Murdock B, Rosner B. Reduction of mortality associated with nosocomial urinary tract infection. Lancet. 1983;1:893–6. DOIPubMedGoogle Scholar
- Huth TS, Burke JP, Larsen RA, Classen DC, Stevens LE. Clinical trial of junction seals for the prevention of urinary catheter-associated bacteriuria. Arch Intern Med. 1992;152:807–12. DOIPubMedGoogle Scholar
- Classen DC, Larsen RA, Burke JP, Stevens LE. Prevention of catheter-associated bacteriuria: clinical trial of methods to block three known pathways of infection. Am J Infect Control. 1990;19:136–42. DOIPubMedGoogle Scholar
- Gabriel MM, Sawant AD, Simmons RB, Hearn DG. Effects of silver on adherence of bacteria to urinary catheters: in vitro studies. Curr Microbiol. 1995;30:1722. DOIPubMedGoogle Scholar
- Saint S, Elmore JG, Sullivan SD, Emerson SS, Koepsell TD. The efficacy of silver alloy-coated urinary catheters in preventing urinary tract infection: a meta-analysis. Am J Med. 1998;105:236–4. DOIPubMedGoogle Scholar
- Maki DG, Stolz SM, Wheeler S, Mermel LA. Prevention of central venous catheterrelated bloodstream infection by use of an antiseptic-impregnated catheter. Ann Intern Med. 1997;127:257–66.PubMedGoogle Scholar
- Nissenkorn I. The intraurethral catheter-three years of experience. Eur Urol. 1993;24:27–30.PubMedGoogle Scholar
- Johnson DE, O'Reilly JL, Warren JW. Clinical evaluation of an external urine collection device for nonambul
Figures
Tables
Cite This ArticleTable of Contents – Volume 7, Number 2—April 2001
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Dennis G. Maki, H4/574 University of Wisconsin Hospital and Clinics, Madison, WI 53792, USA; fax: 608-231-3896
Top