Volume 7, Number 3—June 2001
Dispatch
Nipah Virus Infection in Bats (Order Chiroptera) in Peninsular Malaysia
Abstract
Nipah virus, family Paramyxoviridae, caused disease in pigs and humans in peninsular Malaysia in 1998-99. Because Nipah virus appears closely related to Hendra virus, wildlife surveillance focused primarily on pteropid bats (suborder Megachiroptera), a natural host of Hendra virus in Australia. We collected 324 bats from 14 species on peninsular Malaysia. Neutralizing antibodies to Nipah virus were demonstrated in five species, suggesting widespread infection in bat populations in peninsular Malaysia.
From September 1998 to April 1999, a major outbreak of disease in peninsular Malaysia resulted in the deaths of 105 humans and the slaughter of approximately 1.1 million pigs. The primary causal agent in both pigs and humans, first thought to be the endemic Japanese encephalitis virus, was shown to be a previously undescribed member of the Paramyxoviridae family. Preliminary characterization of a human isolate of the new virus, subsequently named Nipah virus, showed it to have ultrastructural, serologic, antigenic, and molecular similarities to Hendra virus (1-3).
This apparently close phylogenetic relationship focused initial wildlife surveillance on bats (order Chiroptera), particularly pteropid bats (flying foxes), species of which are the probable natural host of Hendra virus in Australia (4-6). Additional support for this targeted approach was provided by the findings of earlier serologic surveillance of flying foxes in Papua New Guinea, in which antibodies neutralizing Hendra virus were found in five of six species tested (Field et al., unpub. data). Malaysia has diverse bat fauna, with at least 13 species of fruit bats (including two species of flying fox) and >60 species of insectivorous bats (7).
We investigated fruit bats (suborder Megachiroptera) and insectivorous bats (suborder Microchiroptera) in peninsular Malaysia for evidence of infection with Nipah virus. Wild boar (Sus scrofa), domestic dogs (Canis lupus) used to hunt wild boar, and rodents (Rattus rattus) trapped on farms with infected pigs were a secondary focus. A parallel study undertook the primary surveillance of rodents, domestic dogs, and other peridomestic species (Mills et al., unpub. data).
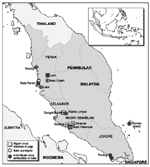
From April 1 to May 7, bats were sampled in 11 primary locations in the states of Perak (n = 6), Selangor (n = 1), Negeri Sembilan (n = 1), and Johore (n = 3) (Figure 1). Most primary locations had more than one sampling site. Locations included but were not restricted to places where Nipah virus-associated disease was reported in pigs. Populations of flying foxes were nonrandomly sampled by shooting foraging or roosting animals. Populations of smaller fruit bats and insectivorous bats were nonrandomly sampled by using mist nets in orchards, oil palm plantations, secondary native vegetation, and residential areas, where bats were reported or observed, where flowering or fruiting trees were observed, and near known or possible roosts. A target of 30 animals per species was set, providing 95% statistical confidence of detecting infection at a minimum population prevalence of 10%, assuming homogeneity of infection across overlapping populations and a test sensitivity and specificity of 100% (8). Blood for serologic examination was also collected from two captive colonies of flying foxes in zoos. In addition to blood, fresh tissue samples of liver, lung, kidney, spleen, heart, and fetus were taken from wild-caught animals, and the carcasses were stored in 10% buffered formalin for reference. Virus isolation was attempted by using Vero E6 cells in a biosafety level 4 laboratory as described (9). All cell harvests were checked for Nipah virus antigens by indirect fluorescence with Nipah hyperimmmune ascitic fluid. Reverse transcriptase-polymerase chain reaction (RT-PCR) was performed on the same tissues, from which RNA was extracted. Virus isolation and RT-PCR were performed on both kidney and spleen from each animal from which tissues had been collected. Additionally, all tissues were blind passaged twice more, and each harvest was tested for viral antigen. RT-PCR used forward and reverse primers designed to amplify a 228-bp region of the N gene.
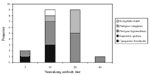
A total of 324 animals from 14 species of bat were sampled, with the target sample size being achieved for five species. Sera were either forwarded directly to the Australian Animal Health Laboratory (AAHL) in Geelong, Australia, or screened by indirect enzyme-linked immunofluorescent assay (ELISA) using Nipah virus antigen at the Veterinary Research Institute in Ipoh, Malaysia, before being forwarded to AAHL. At AAHL, sera were tested by indirect ELISA using Nipah virus antigen and by serum neutralization test (SNT). As the latter is the recognized standard, we used these data in our analysis. Serum neutralization results were obtained for 265 sera, the balance producing toxic reactions (attributed to poor serum sample quality) at a dilution of >1:10. Neutralizing antibodies to Nipah virus were detected in the sera of 21 wild-caught animals from five species (Table): Pteropus hypomelanus (island flying fox), P. vampyrus (Malayan flying fox), Eonycteris spelaea (cave bat), Cynopterus brachyotis (lesser dog-faced fruit bat), and Scotophilus kuhli (house bat). Antibody prevalence among these species was 31%, 17%, 5%, 4%, and 3%, respectively. Titers ranged from 1:5 (n = 2), the lowest dilution tested, to 1:40 (n = 1), median 1:10 (Figure 2). The Nipah virus neutralization titer of the positive control rabbit anti-Hendra virus serum was 1:20. Of the 21 sera neutralizing Nipah virus, only one neutralized Hendra virus, with a titer fourfold less than the corresponding Nipah virus titer. The Hendra virus neutralization titer of the positive control anti-Hendra virus serum was 1:160. All culture harvests were negative for Nipah virus antigen. Two of the tissues yielded cytopathic agents that do not react with either Nipah or Hendra antibodies; these agents are being characterized. All attempts to amplify Nipah virus RNA were also negative.
Wild boar, hunting dogs, and rodents were sampled in Perak state from April 1 to May 7. Wild boar (n = 18) were nonrandomly sampled by shooting in oil palm plantations, secondary native vegetation, national parks, and rural residential areas. Blood samples were also collected from dogs (n = 16) used to hunt wild boar. Rodents (n = 25) were trapped on several farms where pigs were infected. None of the sera from wild boar, hunting dogs, or rodents were positive by indirect ELISA using Nipah virus antigen.
We interpret the presence of neutralizing antibodies to Nipah virus in the identified bat species as evidence of infection with this virus or a cross-neutralizing virus. Cross-neutralization of Nipah antigen by antibodies to Hendra virus was excluded as the cause of the reactivity, and other paramyxoviruses have not demonstrated cross-neutralization with either Hendra (10) or Nipah virus (2). We believe that the presence of anti-Nipah antibodies in a population of P. hypomelanus on the east coast island of Tioman (Figure 1), geographically remote from the west coast foci of Nipah viral disease in pigs, indicates that Nipah virus infection is widespread in flying fox populations in peninsular Malaysia. Ecologically, P. hypomelanus is an island specialist whose mainland foraging is limited to nearby coastal areas (11).
The low neutralizing antibody titers in the positive Malaysian bats were unexpected. In Australia, anti-Hendra virus titers of >1:640 in wild-caught flying foxes (Field et al., unpub. data) and 1:80 in experimentally infected flying foxes (12) have been observed. The absence of high titers in the sampled animals could be explained in several ways: the sample may not be representative of the population; Nipah virus may bind inefficiently to Vero cells used in the neutralization assays; bats' immune response to Nipah virus may be muted as a result of high-level adaptation of the virus to these species; or the antigenic structure of the virus in pigs and humans may differ from that in bats, resulting in less effective neutralization of a test antigen derived from a human isolate. Alternatively, the antibodies detected may be cross-neutralizing antibodies to another related but as yet unidentified virus in bats.
The detection of anti-Nipah virus antibodies in non-pteropid species is notable, although the significance of the finding remains unclear. Limited surveillance of non-pteropid species in Australia for anti-Hendra virus antibodies has not found evidence of infection in these species. Further work is needed to clarify any role of non-pteropid species in the natural history of both viruses.
Isolation of Nipah virus from bats is essential to corroborate the serologic findings and enable comparison of bat isolates with human and pig isolates. However, cell culture of fresh tissue samples from antibody-positive and -negative bat species forwarded to the Centers for Disease Control and Prevention did not produce an isolate reactive with anti-Nipah virus antibodies. All PCR attempts on these tissues were also negative. The tissues submitted (heart, liver, lung, kidney, spleen, fetal) were considered appropriate, as these tissues, as well as white cells, have yielded Hendra virus isolates in naturally infected (13) or experimentally infected (12) flying foxes in Australia. In Malaysia, the period of sampling did not overlap the seasonal gestation of either P. vampyrus or P. hypomelanus. Fetal tissues submitted were from S. kuhli, E. spelaea, C. brachyotis, Taphozous melanopogon, T. saccolaimus, and Rhinolophus affinis.
The wild boar and hunting dog serologic results need to be interpreted in light of the limited sample size, nonrandom sampling, and test methods. Nonetheless, as the behavioral and foraging patterns of wild boar promote contact within and between neighboring populations, the absence of anti-Nipah virus antibodies in the sample supports the absence of established infection in wild boar populations in the areas surveyed. The absence of anti-Nipah virus antibodies in hunting dogs is also consistent with lack of exposure to Nipah virus. A Nipah virus antibody prevalence of 42 (46%) of 92 was identified in domestic dogs sampled near infected pig farms (Mills et al., unpub. data), and if hunting dogs, which have regular contact with the blood, urine, and oronasal secretions of wild boar, were exposed, similar antibody prevalences could reasonably be expected. The negative findings in the rodent sample are consistent with those of the comprehensive parallel survey of rodents (Mills et al., unpub. data).
We report evidence of infection with Nipah virus in four fruit bat species and one insectivorous bat species in peninsular Malaysia. A proposed second phase will describe the occurrence and frequency of infection in the identified Nipah antibody-positive species at additional locations in peninsular Malaysia and in Sabah and Sarawak, Borneo. In addition to successful virus isolation from bats, other proposed research includes retrospective studies of archival specimens, experimental infections of fruit bats, and serologic surveys of other arboreal mammalian species.
Dr. Johara is a senior veterinarian with the Malaysian Department of Veterinary Services. Her research interests include infectious diseases of livestock, infectious zoonotic diseases, animal welfare and wildlife conservation. During the Nipah virus outbreak, she played a key role in the investigation of wildlife species for the origin of Nipah virus.
Acknowledgment
We thank our colleagues from the Malaysian Department of Veterinary Services Veterinary Research Institute at Ipoh for field and laboratory assistance and hospitality; our colleagues from the Malaysian Department of Wildlife and National Parks for their time, knowledge, and field expertise; Craig Smith, Animal Research Institute, Queensland Department of Primary Industries, for technical and laboratory support; Greer Meehan, CSIRO Australian Animal Health Laboratory, for assistance with serology; Lim Boo Liat, formerly of the Malaysian Department of Wildlife and National Parks, for his interest and advice; and village leaders and property owners for their cooperation.
References
- Centers for Disease Control and Prevention. Outbreak of Hendra-like virus--Malaysia and Singapore, 1998-1999. MMWR Morb Mortal Wkly Rep. 1999;48:265–9.PubMedGoogle Scholar
- Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A, Lam SK, Nipah virus: a recently emergent deadly paramyxovirus. Science. 2000;288:1432–5. DOIPubMedGoogle Scholar
- Mohd Nor M, Gan C, Ong B. Nipah virus infection of pigs in peninsular Malaysia. Rev Sci Tech. 2000;19:160–5.PubMedGoogle Scholar
- Young PL, Halpin K, Selleck PW, Field HE, Gravel JL, Kelly MA, Serologic evidence for the presence in Pteropus bats of a paramyxovirus related to equine morbillivirus. Emerg Infect Dis. 1996;2:239–40. DOIPubMedGoogle Scholar
- Field H, Halpin K, Young P. An overview of equine morbillivirus and bat paramyxovirus in Australia. The OIE/FAVA Epidemiology Programme: special session on emerging diseases, Cairns, Australia; 1997 Aug 28; Office International des Epizooties (OIE).
- Williamson MM, Hooper PT, Selleck PW, Gleeson LJ, Daniels PW, Westbury HA, Transmission studies of Hendra virus (equine morbillivirus) in fruit bats, horses and cats. Aust Vet J. 1998;76:813–8. DOIPubMedGoogle Scholar
- Medway L. The wild mammals of Malaya (Peninsular Malaysia) and Singapore. Kuala Lumpur: Oxford University Press; 1978.
- Cannon R. Livestock disease surveys: a field manual for veterinarians. Canberra (Australia): Australian Bureau of Animal Health; 1982.
- Leirs H, Mills JN, Krebs JW, Childs JE, Dudu A, Woollen N, Search for the Ebola virus reservoir in Kikwit, Democratic Republic of the Congo: reflections on a vertebrate collection. J Infect Dis. 1999;179:155–63. DOIPubMedGoogle Scholar
- Murray K, Selleck P, Hooper P, Hyatt A, Gould A, Gleeson L, A morbillivirus that caused fatal disease in horses and humans. Science. 1995;268:94–7. DOIPubMedGoogle Scholar
- Mickleburg S, Hutson A, Racey P. Old world fruit bats: an action plan for their conservation. Gland (Switzerland): International Union for the Conservation of Nature and Natural Resources; 1992.
- Williamson M, Hooper P, Selleck P, Westbury H, Slocombe R. The effect of Hendra virus on pregnancy in fruit bats and guinea pigs. J Comp Pathol. 2000;122:201–7. DOIPubMedGoogle Scholar
- Halpin K, Young P, Field H, Mackenzie J. Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus. J Gen Virol. 2000;81:1927–32.PubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 7, Number 3—June 2001
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Hume Field, Animal Research Institute, Queensland Dept. Primary Industries, LMB 4 Moorooka, 4105, Brisbane, Australia; fax: 61-7-3362-9457
Top