Volume 16, Number 6—June 2010
Dispatch
Xenotropic Murine Leukemia Virus–related Gammaretrovirus in Respiratory Tract
Figure
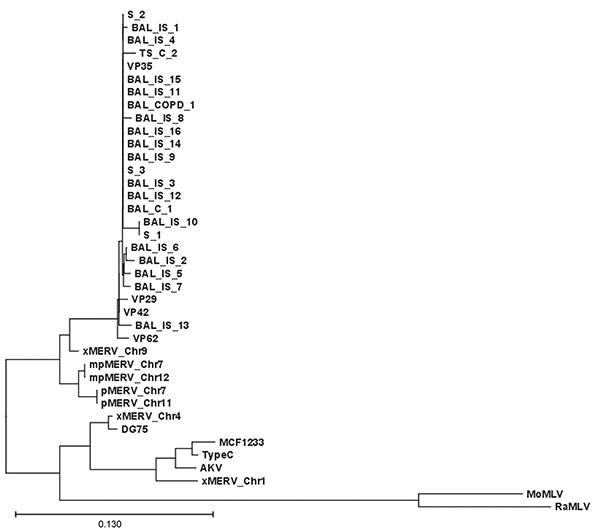
Figure. Xenotropic murine leukemia virus–related gammaretrovirus (XMRV) gag sequences derived from respiratory tract secretions. Phylogenetic tree comparing the 390-nt gag fragment of all respiratory samples of this study with recently published XMRV sequences from patients with familial prostate cancer (1). The edited sequences were aligned with ClustalX version 1.82 (13,14) by using default settings. The tree was generated on the basis of positions without gaps only. Sequences are labeled as X, xenotropic; P, polytropic; mP, modified polytropic; S, sputum, IS, immunosuppression; TS, tracheal secretion; and C, control. Scale bar indicates nucleotide substitutions per position.
References
- Urisman A, Molinaro RJ, Fischer N, Plummer SJ, Casey G, Klein EA, Identification of a novel gammaretrovirus in prostate tumors of patients homozygous for R462Q RNASEL variant. PLoS Pathog. 2006;2:e25. DOIPubMedGoogle Scholar
- Fischer N, Hellwinkel O, Schulz C, Chun FK, Huland H, Aepfelbacher M, Prevalence of human gammaretrovirus XMRV in sporadic prostate cancer. J Clin Virol. 2008;43:277–83. DOIPubMedGoogle Scholar
- Hohn O, Krause H, Barbarotto P, Niederstadt L, Beimforde N, Denner J, Lack of evidence for xenotropic murine leukemia virus–related virus (XMRV) in German prostate cancer patients. Retrovirology. 2009;6:92. DOIPubMedGoogle Scholar
- Stieler KSC, Lavanya M, Aepfelbacher M, Stocking C, Fischer N. Host range and cellular tropism of the human exogenous gammaretrovirus XMRV. Virology. 2010;399:23–30. DOIPubMedGoogle Scholar
- Lombardi VC, Ruscetti FW, Das Gupta J, Pfost MA, Hagen KS, Peterson DL, Detection of an infectious retrovirus, XMRV, in blood cells of patients with chronic fatigue syndrome. Science. 2009;326:530–1. DOIPubMedGoogle Scholar
- Erlwein O, Kaye S, McClure MO, Weber J, Wills G, Collier D, Failure to detect the novel retrovirus XMRV in chronic fatigue syndrome. PLoS ONE. 2010;5:e8519. DOIPubMedGoogle Scholar
- Groom HC, Boucherit VC, Makinson K, Randal E, Baptista S, Hagan S, Absence of xenotropic murine leukaemia virus–related virus in UK patients with chronic fatigue syndrome. Retrovirology. 2010;7:10. DOIPubMedGoogle Scholar
- van Kuppeveld FJ, de Jong AS, Lanke KH, Verhaegh GW, Melchers WJ, Swanink CM, Prevalence of xenotropic murine leukaemia virus–related virus in patients with chronic fatigue syndrome in the Netherlands: retrospective analysis of samples from an established cohort. BMJ. 2010;340:c1018. DOIPubMedGoogle Scholar
- Hong S, Klein EA, Das Gupta J, Hanke K, Weight CJ, Nguyen C, Fibrils of prostatic acid phosphatase fragments boost infections with XMRV (xenotropic murine leukemia virus–related virus), a human retrovirus associated with prostate cancer. J Virol. 2009;83:6995–7003. DOIPubMedGoogle Scholar
- Schlaberg R, Choe D, Brown KR, Thaker HM, Singh IR. XMRV is present in malignant prostatic epithelium and is associated with prostate cancer, especially high-grade tumors. Proc Natl Acad Sci U S A. 2009;106:16351–6. DOIPubMedGoogle Scholar
- Luna LK, Panning M, Grywna K, Pfefferle S, Drosten C. Spectrum of viruses and atypical bacteria in intercontinental air travelers with symptoms of acute respiratory infection. J Infect Dis. 2007;195:675–9. DOIPubMedGoogle Scholar
- Dong B, Kim S, Hong S, Das Gupta J, Malathi K, Klein EA, An infectious retrovirus susceptible to an IFN antiviral pathway from human prostate tumors. Proc Natl Acad Sci U S A. 2007;104:1655–60. DOIPubMedGoogle Scholar
- Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ. Multiple sequence alignment with Clustal X. Trends Biochem Sci. 1998;23:403–5. DOIPubMedGoogle Scholar
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–82. DOIPubMedGoogle Scholar
- Wood KL, Chaiyarit P, Day RB, Wang Y, Schnizlein-Bick CT, Gregory RL, Measurements of HIV viral loads from different levels of the respiratory tract. Chest. 2003;124:536–42. DOIPubMedGoogle Scholar
Page created: February 11, 2011
Page updated: February 11, 2011
Page reviewed: February 11, 2011
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.