Volume 10, Number 7—July 2004
Research
Fluoroquinolone and Other Antimicrobial Resistance in Invasive Pneumococci, Hong Kong, 1995–2001
Cite This Article
Citation for Media
Abstract
We determined the susceptibilities of 265 invasive isolates of pneumococci obtained during 1995 to 2001 in Hong Kong to 11 antimicrobial agents and their serotypes. Overall, 62.6% isolates were susceptible to penicillin, 20% were intermediately resistant, and 17.4% were resistant. The overall prevalence of levofloxacin resistance (MIC >8 μg/mL) was 3.8% but increased to 15.2% among the penicillin-resistant isolates. All levofloxacin-resistant isolates were clonally related; had reduced susceptibility to penicillin, cefotaxime, and clarithromycin; and were derived from adults >50 years of age. Of the penicillin-nonsusceptible pneumococci, 90% were from children <5 years of age, and 54.8% from persons of all ages are of serotypes that are included in the 7-valent pneumococcal conjugate vaccine; 93.5% from children <5 years of age and 93% from persons of all ages are of serotypes that are included in the 23-valent polysaccharide vaccine.
The emergence of antimicrobial resistance in Streptococcus pneumoniae worldwide is an important public health issue because this organism is the leading cause of many infections, particularly community-acquired pneumonia. In many countries, rates of resistance for penicillin, macrolides, and tetracyclines have reached levels of 30% to 40% or higher and are increasing. In recent years, the emergence of fluoroquinolone resistance is being increasingly recognized among multidrug-resistant strains of S. pneumoniae. Hong Kong, Ireland, Canada, and Spain have reported increasing rates of fluoroquinolone resistance among S. pneumoniae (1–3). So far, reports on fluoroquinolone resistance in S. pneumoniae have predominantly involved respiratory tract isolates, and whether this type of resistance is emerging among the invasive isolates is unknown. In this study, we evaluated the comparative activities of five fluoroquinolones against invasive isolates of S. pneumoniae from Hong Kong that were collected during a period when fluoroquinolone resistance had increased rapidly among noninvasive respiratory isolates.
Bacterial Isolates
Stored isolates of S. pneumoniae were obtained from the blood and cerebrospinal fluid (CSF) of patients admitted to five hospitals in Hong Kong during 1995 to 2001. These five hospitals were chosen because they represent the same sentinel network that participated in an earlier study conducted by the same group (4). One hospital did not store the isolates and thus was not included in the present study. The following numbers of isolates were obtained from each of the hospitals: A (140 isolates from 1995 to 2001), B (22 isolates from 1996 to 2001), C (34 isolates from 1997 to 2001), D (64 isolates from 1998 to 2001), and E (5 isolates from 2001). All are public hospitals that provide acute patient care, including all the major specialties. Hospital A is a university teaching hospital with a bone marrow transplant unit, and the others are regional hospitals. Hospitals A and B are located in the same region, and they together serve a population of approximately 1.4 million. Hospitals C, D, and E serve populations of 0.6, 1, and 0.4 million, respectively. Thus, this network together serves approximately 53% of the 6.5 million population in Hong Kong. Isolates included in this study represented all the invasive pneumococcal case-patients with a positive blood or CSF culture in the stated periods. All invasive isolates were tested for antimicrobial susceptibility. Only one isolate from the same patient episode of infection was included. Three patients had two episodes of infections separated by intervals of 3months to 2 years. All isolates were subcultured and reidentified by considering the following characteristics: colony morphologic features, Gram stain results, optochin susceptibility, and bile solubility. Isolates were stored at –20°C until they were tested in batches.
Antimicrobial Agents and Susceptibility Testing
E-test strips of penicillin, amoxycillin (as amoxycillin-clavulanate 2:1), cefotaxime, cefepime, clarithromycin, vancomycin, ciprofloxacin, levofloxacin, sparfloxacin, gatifloxacin, and moxifloxacin were purchased from AB Biodisk, Solna, Sweden. E-test MICs were determined following the manufacturer’s instructions. All susceptibility testing was conducted in a single laboratory at the University of Hong Kong. Test inocula were prepared from pneumococcal colonies grown on sheep blood agar that had been incubated for 20 to 24 h in 5% CO2. Colonies were suspended in 0.9% saline to obtain a suspension equivalent to a 0.5 McFarland standard of turbidity. From this suspension, E-tests were performed on Mueller-Hinton agar with 5% sheep blood (BBL, Becton Dickinson Microbiology Systems, Cockeysville, MD). The plates were incubated at 35°C in 5% CO2 for 20 h to 24 h. MICs falling between two marks on the E-test strip were rounded up to the next higher twofold dilution, as recommended in the instructions. For all MIC determinations, the bacterial inocula were validated by back titration in 10% of the tests to ensure the desired inoculum density. Quality control strains (S. pneumoniae ATCC 49619, Staphylococcus aureus ATCC 29213, and Escherichia coli ATCC 25922) were included with each run. Results were interpreted according to published breakpoints of the National Committee for Clinical Laboratory Standards (5). The term nonsusceptible was used to denote both intermediate and resistant isolates. For ciprofloxacin, the criteria were susceptible, <2 μg/mL; resistant, >4 μg/mL.
Typing of Isolates
All isolates were serotyped by the quellung reaction (6) with sera of various levels of reactivity from the Statens Seruminstitut (Copenhagen, Denmark). The subset of 11 isolates with resistance to ciprofloxacin was examined further by multilocus sequence typing (MLST) and by HinfI restriction analysis of their pbp 2b and 2x genes (7). The well-defined Spanish clones of serotypes 23F and 6B (SP264 ATCC 700669 and GM17 ATCC 700670, respectively) and a strain representative of the fluoroquinolone-resistant variant Hong Kong23F-1 clone were used as controls (4).
Polymerase Chain Reaction (PCR) and DNA Sequencing
The quinolone resistance–determining regions of gyrA, gyrB, parC, and parE were amplified by using primers described previously (8). Nucleotide sequencing was performed by the ByeDye dideoxynucleotide chain termination method (Applied Biosystems, Hong Kong). The sequences of both strands of the amplicons were determined.
Statistical Analysis
Chi-square or Fisher exact test was used for statistical analysis. A p value of <0.05 was considered significant.
Emergence of Fluoroquinolone Resistance among Multidrug-resistant Isolates
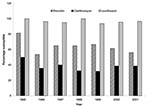
The number of isolates obtained from different age groups was as follows: <2 years (n = 48); 2–5 years (n = 40); 6–17 years (n = 14); 18–49 years (n = 30); 50–64 (n = 27), and >65 years (n = 106). Of the isolates, 256 (96.6%) were from blood, 6 from CSF, and 3 from brain abscess. The susceptibilities of the 265 pneumococcal isolates to 11 antimicrobial agents are summarized in Table 1. The annual susceptibility rates for penicillin, clarithromycin, and levofloxacin are shown in Figure 1. Overall, 166 (62.6%) were penicillin-susceptible, 53 (20%) were penicillin-intermediate, and 46 (17.3%) were penicillin-resistant. Rates of penicillin nonsusceptibility (MIC >0.06 μg/mL) do not differ significantly in the five hospitals (p = 0.1): 35.7% (50 of 140) for laboratory A, 50% (11 of 22) for laboratory B, 32.4% (11 of 34) for laboratory C, 37.5% (24 of 64) for laboratory D, and 60% (3 of 5) for laboratory E.
In children (ages <12 years), the rate of penicillin nonsusceptibility was significantly higher than that in adults (>13 years of age) (48% vs. 30.9%, respectively; p = 0.005). In general, MICs of penicillin were identical or within one dilution difference of that of amoxicillin. For these two penicillins, the MIC50, MIC90, and mode MIC values were identical. On the other hand, MICs of cefotaxime were generally one dilution lower than that of cefepime. High MICs of penicillin (4 μg/mL) and cefotaxime (4 μg/mL) were found in 2 (0.8%) and 1 (0.4%) of 265 isolates, respectively.
Of the 265 isolates, 97 (36.6%) were clarithromycin-susceptible, 1 (0.4%) was clarithromycin-intermediate, and 167 (63%) were clarithromycin-resistant. In most isolates, the resistance was of a high level type. Among 168 clarithromycin-nonsusceptible isolates, 102 (60.7%) had MIC >32 μg/mL, and 78 (46.4%) had MIC >256 μg/mL. Again, clarithromycin resistance rate was higher among children than adults (83 [83%] of 100 vs. 85 [51.5%] of 165, respectively; p < 0.001). The clarithromycin-nonsusceptibility rates in penicillin-susceptible and nonsusceptible isolates were 73 (44%) of 166 and 95 (95.9%) of 99, respectively (p < 0.001).
Overall, 10 (3.8%) of 265 isolates were resistant to levofloxacin. The levofloxacin-resistance rate increased to 15.2% among the penicillin-resistant pneumococcal isolates and was 7.5% among isolates derived from persons >50 years of age. One penicillin-susceptible isolate (S1D3) had a ciprofloxacin MIC of 4 μg/mL. This isolate remained susceptible to levofloxacin. The 10 resistant isolates had a levofloxacin MIC from 16 μg/mL to 32 μg/mL; 8 of these were resistant to gatifloxacin (MIC range, 4−16 μg/mL), and 6 were resistant to moxifloxacin (MIC range, 4−8 μg/mL). All levofloxacin-resistant isolates were also either intermediately resistant or resistant to penicillin (MIC range, 1−4 μg/mL) and clarithromycin (MIC range, 2−>256 μg/mL). All levofloxacin-resistant isolates were from adults (one from the 50-to 64-year age group and nine from >65-year group). Seven were nosocomial infections (having onset >2 days after admission), and three were community-acquired infections. For the levofloxacin-susceptible isolates, the rank order of potency (MIC50/MIC90) was as follows: moxifloxacin (0.125/0.25) > gatifloxacin (0.25/0.25) > sparfloxacin (0.25/0.5) > levofloxacin (1/1) = ciprofloxacin (1/1).
Serotype Distribution of Isolates and Resistance Patterns
Of 265 isolates, 8 isolates could not be typed; 34 different serotypes were identified among the remaining isolates. The serotype distribution of the isolates according to age group of patients and penicillin resistance is shown in Table 2. Serotype 14 was the most common serotype (24.5%). Four serotypes (6B, 14, 19F, and 23F) accounted for 92.9% of all penicillin-nonsusceptble isolates and 84.5% of all clarithromycin-nonsusceptible isolates. The capsular serotypes of the levofloxacin-resistant isolates were 14 (n = 4), 19F (n = 2), and 23F (n = 4). Serotypes included in 7-valent pneumococcal conjugate vaccine formulations (4, 6B, 9V, 14, 18C, 19F, and 23F) comprised 90.4% and 90.6% of penicillin- and clarithromycin-nonsusceptible strains isolated from persons with age <5 years, respectively. Coverage of the 7-valent conjugate vaccine for all isolates from young children (<5 years of age) was 89.7% (79/88). Serotypes included in the 23-valent pneumococcal polysaccharide vaccine accounted for 92.9% of penicillin-nonsusceptible and 91.1% of clarithromycin-nonsusceptible isolates for all ages, respectively.
Molecular Analysis of Fluoroquinolone-resistant Isolates
Molecular analysis of the 11 fluoroquinolone-resistant isolates is summarized in Table 3. Analysis by MLST showed that a single allelic profile (4-4-2-4-4-1-1) or sequence type (ST 81) was shared by all 10 levofloxacin-resistant isolates. Fingerprint patterns after HinfI digestion of the amplified pbp 2x and 2b genes are shown in the Figure 2. One fingerprint pattern for pbp 2b was shared by nine levofloxacin-resistant isolates. The remaining levofloxacin-resistant isolate has a pattern that differed from the major pattern by one band. A single fingerprint pattern for pbp 2x was shared by all 10 levofloxacin-resistant isolates. Both pbp 2b and 2x fingerprint patterns among the levofloxacin-resistant isolates were indistinguishable from that displayed by the Spain23F-1 clone. The remaining ciprofloxacin-resistant, levofloxacin-susceptible strain had distinct pbp 2b and 2x fingerprint patterns. Furthermore, the 10 isolates all had similar pattern of mutations in gyrA, parC, and parE genes. In GyrA, all 10 isolates had a S81F or Y substitution. In ParC, the 10 isolates had at least one amino acid substitution, and 6 isolates had two substitutions, an S79F plus K137N pair. In ParE, one isolate had no substitution. Five isolates had one substitution (I460V), and four had two substitutions (1460V plus D435 or E474K pair). The remaining ciprofloxacin-resistant, levofloxacin-susceptible isolate (S1D3) had a distinct MLST pattern and PBP 2B and 2X gene profiles. This isolate had one substitution in each of ParC and ParE. No strains had substitutions in GyrB.
This study showed that fluoroquinolone resistance among pneumococci that cause invasive infections is emerging. Our finding of a 3.8% resistance rate for levofloxacin is among the highest ever reported in the world and could be attributed in part to suboptimal use of the fluoroquinolones (9). In a case-control study, we have previously shown that chronic obstructive airway disease, nosocomial infection, nursing home residence, and exposure to lesser potent fluoroquinolones were independently associated with fluoroquinolone-resistant Streptococcus pneumoniae (9). Elsewhere, levofloxacin resistance among the invasive pneumococcal isolates were still rare at <1% (10–12). In the United States, Jorgensen et al. recently reported that fluoroquinolone-resistant pneumococci could be also emerging in some of the Active Bacterial Core Surveillance Areas (ABCs). Of 538 invasive pneumococci collected from 1998 to 2000 from California, 3.2% had ciprofloxacin MIC of >4 μg/mL (13). In general, antimicrobial resistance among the pneumococci occurred more frequently among respiratory tract isolates than blood isolates (2). In our previous studies, the fluoroquinolone resistance rate among the respiratory isolates was 5.5% and 13% in 1998 and 2000, respectively (3,4).
Our findings show that invasive pneumococci with fluoroquinolone resistance in this locality were related to the multidrug-resistant Spanish 23F clone. Three different serotypes were identified among the 10 clonally related levofloxacin-resistant isolates, indicating that this clone is evolving by horizontal transfer of the capsular genes. Elsewhere, early evidence suggested that epidemic clones could be playing a role in dissemination of fluoroquinolone resistance. In an analysis of 29 fluoroquinolone-resistant pneumococci, McGee et al. reported that 6 isolates from Ireland and 1 from France were indistinguishable from the Spain9V-3 clone (14). In Birmingham, George et al. recently reported that two fluoroquinolone-resistant variants closely related to the widely distributed penicillin-resistant Spanish9V-3 clone were emerging (15). Furthermore, Alou et al. report that 30% of 82 pneumococci with reduced susceptibility to ciprofloxacin from 20 hospitals in Spain belonged to two internationally spread clones: France9V-3 and Spain23F-1 (16). The emergence of fluoroquinolone resistance among the internationally distributed S. pneumoniae clones is of concern. The Hong Kong experience is an example of how resistance to the fluoroquinolones could evolve rapidly in pneumococci as a result of clonal expansion.
This study found a high rate of macrolide resistance among the invasive pneumococcal isolates, as was reported for noninvasive isolates (4). This circumstance is likely related to the high local use of macrolides and the dissemination of several drug-resistant clones (17). Among invasive isolates, our figure was similar to that reported in Taiwan (72%) but was higher than those reported from the United States (20.4%), Canada (14.8%), or Germany (15.3%) (18–21). Despite early skepticism, increasing evidence shows that in vitro macrolide resistance does result in clinical and microbiologic failures in systemic pneumococcal infection (22,23). Hence, in the empirical treatment of community-acquired pneumonia, our findings imply that monotherapy with a macrolide is not appropriate in this region.
Our data show that 90% or more of the resistant pneumococci that cause invasive infections in persons of all ages belonged to serotypes that are included in the 23-valent pneumococcal polysaccharide vaccine as well as the 7-valent conjugate vaccine. The 7-valent conjugate vaccine is indicated in young children and is highly effective in preventing vaccine serotype-related invasive diseases (24). In the United States, the 7-valent conjugate vaccine was added to the routine schedule in 2000. According to Whitney et al. (25), after the conjugate vaccine was introduced, the rate of invasive disease caused by vaccine and vaccine-related serotypes has markedly declined. The rate of disease caused by strains that were not susceptible to penicillin was 35% lower in 2001 than in 1999. The rate of disease in adults also declined (25). From the results of trials reported so far, the vaccine will likely also reduce carriage of vaccine types of pneumococci (26,27). Hence, resistant pneumococci might diminish as the vaccine becomes more widely available (25,28). The effectiveness of the 23-valent polysaccharide vaccine has not been as dramatic. In older adults, vaccination with the polysaccharide vaccine effectively reduced the rate of bacteremia but not that of nonbacteremic pneumonia (29). The use of the 23-valent pneumococcal vaccine in Hong Kong is low (with estimated coverage of <10% for those >65 years). While data on the efficacy of the 23-valent pneumococcal vaccine are considered insufficient in patients with chronic obstructive pulmonary disease (30), the benefits of vaccinating elderly people with the 23-valent pneumococcal vaccine are clear (31). In view of the findings from this and our previous study that the elderly and persons with chronic obstructive pulmonary disease are at high risk of developing infection by fluoroquinolone-resistant S. pneumoniae, we believe both patient groups in this locality should receive pneumococcal vaccine.
In conclusion, this study reported high rates of fluoroquinolone resistance among multidrug-resistant strains of pneumococci that cause invasive infections among older adults in Hong Kong. Our experience leads us to call for a more prudent use of fluoroquinolones in all clinical settings. Since carriage of pneumococci is common, collateral exposure could occur anytime a person is treated with fluoroquinolones for any infection, including skin, soft tissue, or urinary tract infections. In patients with chronic obstructive pulmonary disease, high-density colonization of the airway is common, which could explain why these patients are at high risk for fluoroquinolone-resistant pneumococci (9). In young children, high numbers of pneumococci are frequently found in the nasopharynx. If fluoroquinolone use is extended from adults to children, who are frequently colonized by the antimicrobial-resistant serotypes, transmission and spread of fluoroquinolone-resistant strains would occur rapidly in the community (32). For pneumonia, all evidence so far indicates that infection caused by pneumococci intermediately resistant to penicillin (MIC <1 μg/L or 2 μg/mL) should respond well to a penicillin given in appropriate doses. In view of this and the emergence of fluoroquinolone resistance in both noninvasive and invasive isolates in this locality, we believe that fluoroquinolones should not be used as first-line treatment in community-acquired pneumonia. In local guidelines, amoxicillin-clavulanate or the combination of amoxicillin and a new macrolide are the recommended first-line drugs for empirical treatment of community-acquired pneumonia in the outpatient setting. As pneumococcal infections become increasing difficult to treat, public health authorities should give priority to pneumococcal vaccination of persons at high risk of acquiring infections by the resistant pneumococci. Since substantial changes can occur in a short period, fluoroquinolone-resistant isolates must be monitored and tracked as part of ongoing and routine pneumococcal surveillance.
Dr. Ho is associate professor in the Department of Microbiology, University of Hong Kong. His research interests include emerging infectious diseases and antimicrobial resistance.
Acknowledgments
We thank Frankie Chow for excellent technical support, Frances Wong for dedicated secretarial assistance, and K.P. Klugmans for kindly providing Spanish clones of serotypes 23F and 6B.
This work was supported by grants from the Health Care and Promotion Fund Committee, the Committee on Research and Conference Grants and Bristol-Myers Squibb (Hong Kong) Ltd.
References
- Ho PL, Cheng VCC. Epidemiology and mechanisms of resistance. In: Ronald AR, Low DE, editors. Milestones in drug therapy: fluoroquinolone antibiotics. Berlin: Birkhauser; 2003. p. 49–72.
- Chen DK, McGeer A, de Azavedo JC, Low DE. Decreased susceptibility of Streptococcus pneumoniae to fluoroquinolones in Canada. Canadian Bacterial Surveillance Network. N Engl J Med. 1999;341:233–9. DOIPubMedGoogle Scholar
- Ho PL, Que TL, Tsang DN, Ng TK, Chow KH, Seto WH. Emergence of fluoroquinolone resistance among multiply resistant strains of Streptococcus pneumoniae in Hong Kong. Antimicrob Agents Chemother. 1999;43:1310–3.PubMedGoogle Scholar
- Ho PL, Yung RW, Tsang DN, Que TL, Ho M, Seto WH, Increasing resistance of Streptococcus pneumoniae to fluoroquinolones: results of a Hong Kong multicentre study in 2000. J Antimicrob Chemother. 2001;48:659–65. DOIPubMedGoogle Scholar
- National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing: eleventh informational supplement. 2001. M100-S11. Wayne (PA): The Committee; 2001.
- Austrian R. The quellung reaction, a neglected microbiologic technique. Mt Sinai J Med. 1976;43:699–709.PubMedGoogle Scholar
- Coffey TJ, Dowson CG, Daniels M, Zhou J, Martin C, Spratt BG, Horizontal transfer of multiple penicillin-binding protein genes, and capsular biosynthetic genes, in natural populations of Streptococcus pneumoniae. Mol Microbiol. 1991;5:2255–60. DOIPubMedGoogle Scholar
- Ho PL, Yam WC, Que TL, Tsang DNC, Seto WH, Ng TK, Target site modifications and efflux phenotype in clinical isolates of Streptococcus pneumoniae from Hong Kong with reduced susceptibility to fluoroquinolones. J Antimicrob Chemother. 2001;47:655–8. DOIPubMedGoogle Scholar
- Ho PL, Tse WS, Tsang KWT, Kwok TK, Ng TK, Cheng VCC, Risk factors for acquisition of levofloxacin-resistant Streptococcus pneumoniae: a case-control study. Clin Infect Dis. 2001;32:701–7. DOIPubMedGoogle Scholar
- Whitney CG, Farley MM, Hadler J, Harrison LH, Lexau C, Reingold A, Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N Engl J Med. 2000;343:1917–24. DOIPubMedGoogle Scholar
- Sahm DF, Peterson DE, Critchley IA, Thornsberry C. Analysis of ciprofloxacin activity against Streptococcus pneumoniae after 10 years of use in the United States. Antimicrob Agents Chemother. 2000;44:2521–4. DOIPubMedGoogle Scholar
- Sahm DF, Karlowsky JA, Kelly LJ, Critchley IA, Jones ME, Thornsberry C, Need for annual surveillance of antimicrobial resistance in Streptococcus pneumoniae in the United States: 2-year longitudinal analysis. Antimicrob Agents Chemother. 2001;45:1037–42. DOIPubMedGoogle Scholar
- Jorgensen JH, Beall B, Hadler J, Harrison LH, Bennett N, Craig A, Invasive disease due to fluoroquinolone-resistant pneumococcus in six surveillance areas, 1998-2000. In: The Third International Symposium on Pneumococci and Pneumococcal Diseases. 5–8 May, 2002. Anchorage, Alaska, USA. Washington: American Society for Microbiology; 2002. Poster 68A.
- McGee L, Goldsmith CE, Klugman KP. Fluoroquinolone resistance among clinical isolates of Streptococcus pneumoniae belonging to international multiresistant clones. J Antimicrob Chemother. 2002;49:173–6. DOIPubMedGoogle Scholar
- Geore RC, Harrison T, Sheppard C, Johnson A, Walker R, Harnett S. Resistance pneumococcal clones and quinolones: an emerging problem. Program and abstracts book: 3rd international symposium on pneumococci and pneumococcal diseases. May 5–8, 2002. Washington: American Society for Microbiology; 2002. p. 26.
- Alou L, Ramirez M, Garcia-Rey C, Prieto J, de Lencastre H. Streptococcus pneumoniae isolates with reduced susceptibility to ciprofloxacin in Spain: Clonal diversity and appearance of ciprofloxacin-resistant epidemic clones. Antimicrob Agents Chemother. 2001;45:2955–7. DOIPubMedGoogle Scholar
- Ip M, Lyon DJ, Yung RW, Chan C, Cheng AF. Evidence of clonal dissemination of multidrug-resistant Streptococcus pneumoniae in Hong Kong. J Clin Microbiol. 1999;37:2834–9.PubMedGoogle Scholar
- Hsueh PR, Teng LJ, Lee LN, Yang PC, Ho SW, Luh KT. Extremely high incidence of macrolide and trimethoprim-sulfamethoxazole resistance among clinical isolates of Streptococcus pneumoniae in Taiwan. J Clin Microbiol. 1999;37:897–901.PubMedGoogle Scholar
- Hyde TB, Gay K, Stephens DS, Vugia DJ, Pass M, Johnson S, Macrolide resistance among invasive Streptococcus pneumoniae isolates. JAMA. 2001;286:1857–62. DOIPubMedGoogle Scholar
- Reinert RR, Al Lahham A, Lemperle M, Tenholte C, Briefs C, Haupts S, Emergence of macrolide and penicillin resistance among invasive pneumococcal isolates in Germany. J Antimicrob Chemother. 2002;49:61–8. DOIPubMedGoogle Scholar
- Weiss K, Guilbault C, Cortes L, Restieri C, Low DE. Genotypic characterization of macrolide-resistant strains of Streptococcus pneumoniae isolated in Quebec, Canada, and in vitro activity of ABT-773 and telithromycin. J Antimicrob Chemother. 2002;50:403–6. DOIPubMedGoogle Scholar
- Jacobs MR. In vivo veritas: in vitro macrolide resistance in systemic Streptococcus pneumoniae infections does result in clinical failure. Clin Infect Dis. 2002;35:565–9. DOIPubMedGoogle Scholar
- Lonks JR, Garau J, Gomez L, Xercavins M, Ochoa-de-Echaguen A, Gareen IF, Failure of macrolide antibiotic treatment in patients with bacteremia due to erythromycin-resistant Streptococcus pneumoniae. Clin Infect Dis. 2002;35:556–64. DOIPubMedGoogle Scholar
- Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19:187–95. DOIPubMedGoogle Scholar
- Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–46. DOIPubMedGoogle Scholar
- Mbelle N, Huebner RE, Wasas AD, Kimura A, Chang I, Klugman KP. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J Infect Dis. 1999;180:1171–6. DOIPubMedGoogle Scholar
- Dagan R, Givon-Lavi N, Zamir O, Sikuler-Cohen M, Guy L, Janco J, Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending day care centers. J Infect Dis. 2002;185:927–36. DOIPubMedGoogle Scholar
- Givon-Lavi N, Fraser D, Porat N, Dagan R. Spread of Streptococcus pneumoniae and antibiotic-resistant S. pneumoniae from day-care center attendees to their younger siblings. J Infect Dis. 2002;186:1608–14. DOIPubMedGoogle Scholar
- Jackson LA, Neuzil KM, Yu O, Benson P, Barlow WE, Adams AL, Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med. 2003;348:1747–55. DOIPubMedGoogle Scholar
- Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–76.PubMedGoogle Scholar
- Hedlund J, Christenson B, Lundbergh P, Ortqvist A. Effects of a large-scale intervention with influenza and 23-valent pneumococcal vaccines in elderly people: a 1-year follow-up. Vaccine. 2003;21:3906–11. DOIPubMedGoogle Scholar
- Mandell LA, Peterson LR, Wise R, Hooper D, Low DE, Schaad UB, The battle against emerging antibiotic resistance: should fluoroquinolones be used to treat children? Clin Infect Dis. 2002;35:721–7. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 10, Number 7—July 2004
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Pak-Leung Ho, Division of Infectious Diseases, Department of Microbiology, Queen Mary Hospital, The University of Hong Kong, Pokfulam Road, Pokfulam, Hong Kong Special Administrative Region, China; fax: 852−2855−1241
Top