Volume 15, Number 4—April 2009
Dispatch
Isolation of Genotype V St. Louis Encephalitis Virus in Florida
Cite This Article
Citation for Media
Abstract
We isolated and characterized St. Louis encephalitis virus (SLEV) from cloacal swabs of naturally exposed adult sentinel chickens in 2006. Phylogenetic analysis of SLEV strains isolated in Florida indicated that Brazilian SLEV circulated in 1972 and 2006; lineages were VA and VB.
In North America, before the introduction of West Nile virus (WNV; Flavivirus, Flaviviridae) in 1999, St. Louis encephalitis virus (SLEV; Flavivirus, Flaviviridae) was the most important agent of epidemic viral encephalitis (1). SLEV activity is restricted to the Western Hemisphere and outbreaks have occurred in North America since 1933 (2). The recent cocirculation of these closely related flaviviruses has raised the possibility that competitive pressures might alter the transmission cycle of WNV, SLEV, or both (3,4).
In Florida, periodic SLEV outbreaks since the 1950s led to the formation of an arbovirus surveillance program (5), anchored by the Florida Sentinel Chicken Arboviral Surveillance Network (6). SLEV is maintained in a mosquito-bird-mosquito cycle; amplification occurs in peridomestic birds and Culex spp. mosquitoes (7). Chickens are chosen as sentinels because they are susceptible to infection and develop antibodies after exposure (seroconversion) (8).
We isolated SLEV from naturally infected adult chickens and compared it with previously isolated strains. The envelope region of viral isolates was analyzed because of its biological importance and high immunogenicity in the host (9).
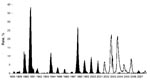
In Florida, SLEV transmission is sporadic with periods of low (enzootic) and high (epidemic) activity. SLEV was detected by sentinel chickens every year before introduction of WNV (1988–2007) (Figure). Since 2001, limited SLEV activity has been reported (10); SLEV may be in a natural decline, or transmission of WNV may influence SLEV cycles, as has been suggested in California (4).
In 2006, a total of 2,901 adult sentinel chickens were maintained at 275 sites of potential enzootic arbovirus transmission in 34 Florida counties. Blood was collected weekly from each chicken during peak transmission months (July–December) and tested with hemagglutination inhibition assay, immunoglobulin M antibody-capture ELISA, or plaque reduction neutralization test, as previously described (11). Sites with confirmed SLEV seroconversions were targeted for sample collection. For the first time since 2001, SLEV sentinel seroconversions (n = 40) exceeded WNV seroconversions (n = 30) (10).
In central and south Florida, 5 partner agencies targeted a subset (n = 15) of sentinel chicken sites with recent confirmed arbovirus transmission activity for cloacal swab collection from 95 chickens. During the weekly scheduled bleeding of the flocks, 1,338 cloacal swabs were collected in viral culturettes (Becton Dickinson, Franklin Lakes, NJ, USA); 529 swabs were retrospectively processed for molecular detection assays and virus isolation in Vero cells, as previously described (12). Viral RNA was extracted from cloacal swabs and first-passage cell cultures and amplified with real-time reverse transcription–PCR (RT-PCR) TaqMan assays for WNV and SLEV, as previously described (13). Two SLEV strains, FL06-S569 and FL06-S650, were detected by RT-PCR and cultured in Vero cells. Fourteen additional SLEV strains were obtained from the Florida Department of Health, Bureau of Laboratories–Tampa archive for phylogenetic analysis (Table).
To characterize SLEV strains, we amplified the envelope region using previously described primers (9) and the SuperScript III 1-step RT-PCR system (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. Sequences were aligned by using ClustalW 1.6 and phylogenetic trees drawn by using the maximum parsimony method, with 1,000 bootstrap replicates, in MEGA 4.0 software (14), including 60 other SLEV envelope sequences available in GenBank (9,15) and 4 related flavivirus outgroups (accession nos.: WNV NY99, AF196835; Japanese encephalitis virus, EF571853; Kunjin virus, AY274505; Murray Valley encephalitis virus, AF161266).
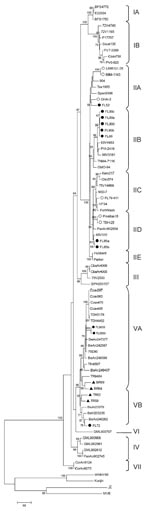
The phylogenetic tree places FL06-S569 and FL06-S650 into genotype VA (Appendix Figure). This analysis further supports classification of SLEV into 7 lineages and 13 clades (IA-IB, IIA-IIE, III, IV, VA-VB, VI, VII), as previously suggested (9). FL06-S569 and FL06-S650 share 98% sequence identity with SLEV strains from South America, including Brazil (BeAn247377, BeAn242587) and Peru (75D90). Two nucleotide mismatches (silent transition mutations at positions 1083, 1404) were noted in the envelope region within the FL06-S569 and FL06-S650 isolates.
Envelope gene sequences were previously published for 6 Florida strains (9), and 9 additional archived Florida isolates were analyzed for this study. Reference strain FL72-M022 was isolated from an opossum from the Florida panhandle in 1972. FL72-M022 shares 97%–98% sequence identity with strains from Brazil (BeAn246262, BeAr23379, and BeH203235) and is placed in genotype VB. In contrast, SLEV reference strains isolated in Florida during 1952 and 1985 share 97%–99% homology with strains collected in Tampa Bay during 1962 (TBH-28, GHA-3) and in Mexico (65V310). The last large outbreak of SLEV in Florida occurred during 1990. Envelope sequence analysis demonstrated that strains isolated during 1989 and 1990 shared 98% homology with USA (V 2380-42), Guatemala (78A28), Tennessee (TNM 4-711), or Texas strains (83V4953, PVI-2419, 98V3181).
Despite detection of SLEV after the introduction of WNV, SLEV had not recently been cultured by existing statewide surveillance methods in Florida (10). Experimental evidence suggests that WNV cross-protective immunity in wild bird species may limit subsequent SLEV infections (3). In 2006, sentinel seroconversions supported this hypothesis; limited WNV activity may have enabled increased transmission of SLEV during the fall (Figure).
Partner agencies in the Florida Arbovirus Surveillance Network used a targeted strategy to preferentially sample sentinels located in “hot zones” of SLEV transmission activity for virus isolation and molecular analysis. Sequence analysis of reference strains and the 2006 SLEV isolates has shown the circulation of genotype V SLEV strains in Florida. The 2006 isolates do not represent a recent extension of the geographic range of strains of SLEV from Brazil because 1 genotype V strain was also collected during field studies in 1972. Instead, they support periodic circulation and maintenance of South American SLEV genotypes in Florida, where the diverse ecosystem may allow for evolution of the virus and periodic seeding of SLEV into the United States where the human population may have no immunity to the virus.
On the basis of placement into multiple lineages (IIA-IID, VA, VB) (Appendix Figure), our data support the hypothesis that persistence of SLEV in Florida may differ from its activity in other regions of the United States. For example, the same or highly similar strains of SLEV can be locally maintained for more than a decade in California and Texas (15), whereas genetically similar strains of SLEV appear to be infrequently isolated, or maintained at levels below detection, over extended periods in Florida. Our findings suggest periodic introduction of different SLEV genotypes to Florida from the eastern United States and other countries (Mexico, Panama, and Brazil), with distinct North American (lineage II) genotypes isolated in epidemic years. The role of South American genotypes in enzootic or epidemic cycles of SLEV is unknown. In Florida, only the detection of North American genotypes has previously been reported (9,15), but the isolation of South American strains in 1972 and 2006 suggests a mechanism for the continued maintenance of SLEV. Further isolation and characterization of SLEV strains is needed to improve understanding of the mechanism(s) that favor the amplification of North vs. South American genotypes in Florida.
Dr. Ottendorfer is a postdoctoral scientist in the department of Global Health at the University of South Florida. Her research interests include surveillance, molecular epidemiology, and ecology of arboviruses.
Acknowledgments
We thank the dedicated people at county mosquito-control districts, the Bureau of Laboratories–Tampa, and Jonathan Day for their contributions to this project.
This project was supported by Centers for Disease Control and Prevention (CDC) Grant/Cooperative Agreement no. U38/CCU423095 and by grants from CDC and the National Institute of Allergy and Infectious Diseases (projects no. R01 CI000226 and R01AI049724).
References
- Lindenbach BD, Rice CM. Flaviviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Field’s virology, 4th ed., vol. 1. Philadelphia: Lippincott Williams & Wilkins; 2001. p. 991–1041.
- Burke DS, Monath TP. Flaviviruses. In: Knipe DM, Howley PM, eds. Field’s virology, 4th ed., vol. 1. Philadelphia: Lippincott Williams & Wilkins; 2001. p. 1043–125.
- Fang Y, Reisen WK. Previous infection with West Nile or St. Louis encephalitis viruses provides cross protection during reinfection in house finches. Am J Trop Med Hyg. 2006;75:480–5.PubMedGoogle Scholar
- Reisen WK, Lothrop HD, Wheeler SS, Kennsington M, Gutierrez A, Fang Y, Persistent West Nile virus transmission and the apparent displacement St. Louis encephalitis virus in southeastern California, 2003–2006. J Med Entomol. 2008;45:494–508. DOIPubMedGoogle Scholar
- Bigler BS. St. Louis encephalitis. 1999 [cited 2008 Jul 1]. Available from http://www.doh.state.fl.us/disease_ctrl/epi/htopics/reports/slepres2.pdf
- Nelson DB, Kappus KD, Janowski HT, Buff E, Wellings FM, Schneider NJ. St. Louis encephalitis—Florida 1977. Patterns of a widespread outbreak. Am J Trop Med Hyg. 1983;32:412–6.PubMedGoogle Scholar
- Day JF. Predicting St. Louis encephalitis virus epidemics: lessons from recent, and not so recent, outbreaks. Annu Rev Entomol. 2001;46:111–38. DOIPubMedGoogle Scholar
- Reisen WK, Presser SB, Lin J, Enge B, Hardy JL, Emmons RW. Viremia and serological responses in adult chickens infected with western equine encephalomyelitis and St. Louis encephalitis viruses. J Am Mosq Control Assoc. 1994;10:549–55.PubMedGoogle Scholar
- Kramer LD, Chandler LJ. Phylogenic analysis of the envelope gene of St. Louis encephalitis virus. Arch Virol. 2001;146:2341–55. DOIPubMedGoogle Scholar
- Florida Department of Health. Arbovirus surveillance: annual and laboratory reports, 1999–2007 [cited 2008 Jul 1]. Available from http://www.doh.state.fl.us/ENVIRONMENT/community/arboviral/survey-info.htm
- Blackmore CG, Stark LM, Jeter WC, Oliveri RL, Brooks RG, Conti LA, Surveillance results from the first West Nile virus transmission season in Florida, 2001. Am J Trop Med Hyg. 2003;69:141–50.PubMedGoogle Scholar
- Ciota AT, Lovelace AO, Ngo KA, Le AN, Maffei JG, Franke MA, Cell-specific adaptation of two flaviviruses following serial passage in mosquito cell culture. Virology. 2007;357:165–74. DOIPubMedGoogle Scholar
- Lanciotti RS, Kerst AJ. Nucleic acid sequence-based amplification assays for rapid detection of West Nile and St. Louis encephalitis viruses. J Clin Microbiol. 2001;39:4506–13. DOIPubMedGoogle Scholar
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–9. DOIPubMedGoogle Scholar
- May FJ, Li L, Zhang S, Guzman H, Beasley DW, Tesh RB, Genetic variation of St. Louis encephalitis virus. J Gen Virol. 2008;89:1901–10. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleTable of Contents – Volume 15, Number 4—April 2009
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Christy L. Ottendorfer, University of South Florida, 3720 Spectrum Blvd, IDRB, Rm 304, Tampa, FL 33612, USA
Top