Volume 16, Number 11—November 2010
Research
Comparison of 3 Infrared Thermal Detection Systems and Self-Report for Mass Fever Screening
Cite This Article
Citation for Media
Abstract
Despite limited evidence regarding their utility, infrared thermal detection systems (ITDS) are increasingly being used for mass fever detection. We compared temperature measurements for 3 ITDS (FLIR ThermoVision A20M [FLIR Systems Inc., Boston, MA, USA], OptoTherm Thermoscreen [OptoTherm Thermal Imaging Systems and Infrared Cameras Inc., Sewickley, PA, USA], and Wahl Fever Alert Imager HSI2000S [Wahl Instruments Inc., Asheville, NC, USA]) with oral temperatures (>100°F = confirmed fever) and self-reported fever. Of 2,873 patients enrolled, 476 (16.6%) reported a fever, and 64 (2.2%) had a confirmed fever. Self-reported fever had a sensitivity of 75.0%, specificity 84.7%, and positive predictive value 10.1%. At optimal cutoff values for detecting fever, temperature measurements by OptoTherm and FLIR had greater sensitivity (91.0% and 90.0%, respectively) and specificity (86.0% and 80.0%, respectively) than did self-reports. Correlations between ITDS and oral temperatures were similar for OptoTherm (ρ = 0.43) and FLIR (ρ = 0.42) but significantly lower for Wahl (ρ = 0.14; p<0.001). When compared with oral temperatures, 2 systems (OptoTherm and FLIR) were reasonably accurate for detecting fever and predicted fever better than self-reports.
Advancements in transportation coupled with the growth and movement of human populations enable efficient transport of infectious diseases almost anywhere in the world within 24 hours (1). This recognition has prompted the evaluation of rapid mass screening methods to delay the importation of infection into healthcare settings, communities, and countries (1–4). Because fever is a common indicator of many infectious diseases, the rapid identification of fever is a major component of screening efforts. Such screening was used by many countries during the severe acute respiratory syndrome outbreak in 2003 and the influenza A pandemic (H1N1) 2009 outbreak (2,3,5–8). Despite widespread implementation of fever screening, its value for detecting highly communicable diseases has mainly been established through mathematical modeling rather than through studies in humans (9,10).
One approach to fever screening is to simply ask persons if they have a fever. In healthcare settings, this information is routinely obtained in the chief complaint or review of symptoms and in some situations by querying persons as they enter the facility (11). In travel settings, many countries have used a written health declaration to screen travelers arriving at international ports of entry (2). However, limited information exists on the accuracy of self-reported fever, which is biased by its subjective nature and reliance on travelers’ awareness of fever status and willingness to report (12,13). Indeed, a clinical trial suggested that traditional thermometry is superior to self-reported fever for identifying patients with seasonal influenza (14). However, traditional thermometry methods are time-consuming and require close contact with potentially infectious patients.
Infrared thermal detection systems (ITDS) offer a potentially useful alternative to contact thermometry. This technology was used for fever screening at hospitals, airports, and other mass transit sites during the severe acute respiratory syndrome and influenza A pandemic (H1N1) 2009 outbreaks (2,3,5–8,15). ITDS appeared to enable early detection of febrile persons entering healthcare facilities, where the undetected introduction of communicable diseases can lead to outbreaks among patients and staff (5,16–18).
Although ITDS have the potential to serve as rapid, noninvasive screening tools for detecting febrile persons, previous studies provide conflicting information about their utility for mass fever screening (15,16,19–25). In addition, there are few published comparisons of the efficacy of different ITDS and their suitability for mass fever screening (19). Finally, no studies on the relative accuracy of self-reported fever and ITDS for fever screening or the value of combining these 2 methods have been published. These questions and the potential need to rapidly screen for fever during an emerging pandemic prompted us to conduct this study to validate different ITDS temperatures and self-reported fevers with oral temperatures.
Study Setting
A cross-sectional study comparing 3 ITDS was conducted in 3 urban tertiary-care hospital emergency departments in the United States: Albuquerque, New Mexico; Atlanta, Georgia; and Chicago, Illinois. Emergency departments were selected as the evaluation setting because of a potential high prevalence of fever compared with its prevalence in healthy populations and the routine measurement of each patient’s oral temperature. The 3 hospitals were selected because of their estimated patient volume of >200 patients per day.
Human Subject Research Protections
The study was approved by the Institutional Review Board (IRB) of the Centers for Disease Control and Prevention (CDC) and the IRBs of the hospitals in Atlanta and Chicago. The Albuquerque hospital’s IRB reviewed the protocol but deferred to CDC’s IRB for approval.
Device Selection
ITDS were selected for evaluation through a competitive bidding process. Selection criteria included specifications suitable for fever screening: view field captures human heights (0.5–2.5 meters), temperature discrimination <0.2°C, smallest available sensor temperature range encompassing human temperatures (–40°C to 120°C), tripod/stationary mount, operational distance >2 meters, internal/external calibration standards, temperature capture time <1 second, and price <$25,000. Of 6 devices submitted to CDC, 3 met the above criteria and were selected for testing: the FLIR ThermoVision A20M (FLIR Systems Inc., Boston, MA, USA), the OptoTherm Thermoscreen (OptoTherm Thermal Imaging Systems and Infrared Cameras Inc., Sewickley, PA, USA), and the Wahl Fever Alert Imager HSI2000S (Wahl Instruments Inc., Asheville, NC, USA). Manufacturers provided training and consultation on the assembly and operation of the ITDS per company practices but were otherwise uninvolved in the study.
Participants and Eligibility
Adults (>18 years of age) were recruited consecutively among patients who sought care at the emergency departments of 1 hospital in each city: Chicago (September 15–29, 2008), Atlanta (October 6–24, 2008), and Albuquerque (February 17–26, 2009). Patients were approached after they had been registered in the emergency department from 7:00
Sample Size
We estimated that 61 febrile patients were necessary to evaluate the sensitivity of ITDS for fever detection (assumed to be 80% from previous research) to within ±10% with 95% confidence. With an estimated fever prevalence of 2% among a population of patients at emergency departments, a total sample size of ≈3,000 patients was needed for the study.
Temperature Measurements
The 3 ITDS were positioned at the optimal distance (2–3 m) from each participant as recommended by the manufacturers. Each ITDS camera field of view was preset to capture the patient’s face and neck. Participants were asked to remove eyeglasses and hats and instructed to stand facing the cameras until temperature measurements from all 3 devices had been recorded.
To account for ambient temperature, the Wahl device was manually calibrated on each morning before data collection, per manufacturer recommendation. In Albuquerque, where room temperatures varied during the day, the Wahl was additionally calibrated after noticeable changes in ambient temperature. The OptoTherm and FLIR have automated calibration systems to adjust for ambient conditions, diurnal variations in temperature, and thermal drift and therefore did not require manual calibration.
Unadjusted skin temperatures detected by ITDS were included in the analysis to enable direct comparison with oral temperature measurements. The FLIR and Wahl cameras did not display fixed temperature readings but rather readings that fluctuated by tenth of a degree increments. For these 2 cameras, operators recorded the highest temperature displayed for each person. Measurements recorded by the FLIR during periods when the camera was not properly focused were excluded from the analysis.
Oral temperatures were measured by clinical staff using a DinaMap ProCare digital thermometer (General Electric Company, Freiburg, Germany) in Albuquerque and Atlanta and a Welch Allyn SureTemp Plus 692 Electronic Thermometer (Welch Allyn Inc, San Diego, CA, USA) in Chicago, per each hospital’s established patient care standard. ITDS temperature measurements were taken either immediately after (Chicago and Atlanta) or just before (Albuquerque) each oral measurement. Confirmed fever was defined as an oral temperature >100°F (>37.8°C). Room temperatures were recorded hourly by using a standard digital room thermometer.
Patient Self-Reports
Upon enrollment, patients were asked, “Do you feel like you have a fever now?” (self-reported fever) and whether they had taken medication for pain or fever (analgesic or antipyretic drugs) in the previous 8 hours. When needed, patients were given examples of trade and generic names of common antipyretic drugs. Their responses, along with each patient’s age and sex, date, and time of temperature measurement were recorded.
Data Analysis
Symptom questionnaire responses, oral temperature measurements, and ITDS-recorded data were entered into an Excel (Microsoft Corp., Redmond, WA, USA) database and analyzed by using SAS Version 9.2 (SAS Institute Inc, Cary, NC, USA). Patient responses of “Don’t know” to the question, “Do you feel like you have a fever now?” were analyzed as “No.” ITDS and oral temperature measurements were compared by using descriptive statistics and bivariate analysis (χ2 tests, t tests, and correlations). Generalized linear modeling was used to investigate the effects of covariates and potential confounders (age, sex, recent antipyretic use, study site, self-reported fever, time of day, and room temperature) on temperature measurements and to identify factors that influenced the difference between oral and ITDS temperature measurements, given site-specific effects.
Sensitivity (the proportion of those with confirmed fever who were identified as febrile by ITDS) and specificity (the proportion of those without confirmed fever who were identified as nonfebrile by ITDS) were calculated and used to plot the receiver operating characteristic (ROC) curves for all possible fever temperature thresholds on each ITDS. Optimal ITDS fever thresholds were defined as the temperature that yielded the highest combined sensitivity and specificity for fever detection for each device as determined by the ROC curves. Positive predictive value (PPV), the proportion of patients identified as febrile by ITDS who had a confirmed fever by oral temperature, was compared with self-report. The accuracies (sum of sensitivity and specificity) of the following 3 fever screening methods were compared by using oral thermometry as reference: 1) self-reported fever, 2) ITDS at optimal fever detection threshold, and 3) combination of ITDS and self-reported fever with a positive result on either method considered a fever.
Of 3,345 eligible patients, we enrolled a total of 2,873 (85.9%): 1,511 (52.6%) in Chicago, 1,040 (36.2%) in Atlanta, and 322 (11.2%) in Albuquerque. The remaining 472 (14.1%) patients refused to participate. Men accounted for 1,514 (52.7%) participants; the mean age was 42 years (range 18–92 years). The mean oral temperature was 97.9°F (range 92.8°F–104.4°F); 64 (2.2%) patients had confirmed fever, including 48 (10.1%) of 476 patients reporting fever. Antipyretic or analgesic drug use within 8 hours was reported by 1,121 (39.0%) patients, including 225 (45.8%) who self-reported fever and 39 (60.9%) who had confirmed fever.
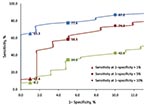
Correlations of ITDS and oral temperatures were similar for OptoTherm (ρ = 0.43) and FLIR (ρ = 0.42) but significantly lower for Wahl (ρ = 0.14; p<0.001). The areas under the ROC curves (AUC) for OptoTherm (96.0%) and FLIR (92.0%) were not significantly different but were significantly greater than the AUC of Wahl (78.2%; p<0.001; Figure 1). At their respective optimal threshold temperatures, sensitivities of fever detection of the 3 ITDS were not significantly different from each other, but specificities and PPVs of OptoTherm and FLIR were significantly higher than those of Wahl (Table 1; p<0.001). At fixed specificities, the sensitivities of each ITDS varied (Figure 2).
Compared with oral thermometry, sensitivity for self-reported fever was 75%, specificity was 84.7%, and PPV was 10.1%. Sensitivities of the 3 ITDS at their respective optimal thresholds did not differ significantly from that of self-reported fever (Table 1). However, specificities and PPVs of OptoTherm and FLIR at optimal thresholds were significantly greater than those of self-reported fever (p<0.001 for both comparisons), and specificity and PPV of Wahl were significantly lower than those of self-reported fever (p<0.001). The addition of self-report decreased the accuracy of fever detection at optimal thresholds for FLIR and OptoTherm (increase in sensitivity was less than decrease in specificity) but improved accuracy for Wahl with a greater increase in sensitivity than the decrease in specificity (Table 1). Conversely, adding OptoTherm or FLIR temperature measurements to self-reported fever increased accuracy, but adding Wahl temperature measurements decreased accuracy (Table 1).
Bivariate analyses revealed higher oral and ITDS temperatures among younger patients and later in the day (Table 2). Oral temperatures were higher in women, and ITDS temperature measurements were higher in men. ITDS temperature measurements increased with increasing room temperatures. Temperatures detected by oral thermometers, OptoTherm, and FLIR were higher in patients who reported recent antipyretic or analgesic drug use.
When we controlled for study site, multivariate analyses showed that 2 variables (sex and room temperature) were most strongly (p<0.001) associated with the size of the gap between oral and ITDS temperature measurements (Table 3). Smaller differences between ITDS and oral temperatures were found among men than among women. Differences between ITDS and oral temperatures became smaller with increasing room temperatures and as the day progressed (with the exception of FLIR). Site-specific effects indicated that, on average, differences between ITDS and oral temperatures were smaller among participants from Albuquerque and Atlanta than among those from Chicago. With the exception of Wahl measurements, the difference between ITDS and oral temperatures was greater in older patients. Differences between oral and OptoTherm temperatures tended to be smaller for those reporting antipyretic drug use.
Our evaluation of 3 ITDS in emergency department settings found that the FLIR and OptoTherm reliably identified elevated body temperatures. The high AUCs for these 2 systems suggest that they can differentiate between febrile and afebrile persons with relatively high sensitivity and specificity at an optimal fever cutoff. The relatively high correlation with oral temperature measurement also supports the utility of these 2 ITDS, which predicted fever better than self-reports of patients and more accurately alone than in combination with self-reported fever.
Our study is one of few that simultaneously examined the effects of multiple external and internal factors (age, sex, time of day, room temperature, and antipyretic drug use) on ITDS and oral temperature measurement accuracy. We found that ITDS and oral temperature measurements were strongly influenced by site and time of day, which may be a real effect or a result of variations in oral measurement techniques. The effects of age and time of day on body temperature found in this study have been well established by previous research (26–28). We observed strong associations between ITDS and room temperatures. Similar observations with room temperatures and extended exposure to hot or cold environments have been reported (22,25,29,30). The unexpected association between higher temperature measurements (oral and OptoTherm) and recent antipyretic drug use may result from patients with higher fevers taking antipyretic drugs, inadequate antipyretic drug dosage, or both. The finding that men had relatively higher ITDS measurements than women has not been previously reported and may be because of differences in facial hair, use of cosmetics, or subcutaneous fat composition (31). Similar associations across multiple ITDS underscore the strength of these findings. By controlling for these covariates, we were able to measure the relationship between ITDS and oral temperatures with greater precision.
Although the sensitivity, specificity, and AUC of the devices we tested were similar to those found in previous studies, we observed a higher correlation between ITDS temperature measurements and confirmatory temperature measurements (15,16,19–25). Several factors may have contributed to these differences. The higher correlation between ITDS and body temperatures reported here may be related to the use of oral temperature measurement as reference. Although oral temperature measurements better reflect core temperatures than infrared tympanometric measurements, most previous investigations of ITDS have used the latter as reference (19,23,24,32–35). The preference for oral temperatures as reference is supported by an evaluation of methods for measuring body temperature conducted by the American College of Critical Care Medicine and the Infectious Diseases Society of America; researchers found that rectal temperatures were the most accurate of the peripheral thermometry methods, followed by oral, tympanic, and axillary temperature measurements, respectively (32).
Many types of ITDS are available, ranging from inexpensive hand-held point-and-shoot devices with laser sighting to hand-held cameras with light-emitting diode displays, wall-mounted cameras, and portable cameras on tripods such as the ones used in this study (19,23,29). To maximize potential efficacy, we evaluated technically advanced ITDS that were recently developed for human temperature detection. Other studies used more basic systems and did not compare different devices. Although the costs of the OptoTherm and FLIR were comparable at $22,000 and $16,000 per system, respectively, the Wahl was relatively less expensive ($8,000). Testing 3 different models at various price ranges allowed us to demonstrate substantial differences among ITDS. These differences are likely to affect their sensitivity and utility for fever screening. The systems used in this study require the person to stand in front of the camera for ≈2–3 seconds to capture a temperature. Other differences, such as moving persons, could have further affected the sensitivity of ITDS for fever detection.
Although addition of a health declaration form would allow screening to also consider recent travel history, previous fever, and other symptoms or illness exposures, health declarations have variable compliance rates and depend on a person’s ability to understand questions and accurately assess symptoms as well as willingness to report (12,13,36,37). In our study, in which patients had no disincentive to report, we found that one fourth of febrile patients did not report having fever, which suggests true unawareness of fever among some persons. Only one tenth of those who reported having a fever were actually found to be febrile. Our results, therefore, probably underestimated the benefit of ITDS over self-reports of fever. In other settings, ill persons may be less likely to report symptoms for fear of adverse consequences such as travel delays, involuntary isolation of ill persons, or quarantine of exposed contacts. In settings such as travel sites (e.g., airports) and the workplace, ITDS could provide an objective means for the mass detection of fever as part of a comprehensive public health screening strategy because ITDS had greater accuracy than self-reports.
Mass health screening during a pandemic will certainly be influenced by several other factors, including perceived and actual pandemic severity, as well as the potential consequences of illness detection, either negative or positive, which can affect the sensitivity of screening that uses self-report. If being detected as febrile is perceived as harmful, travelers may hide their symptoms (12). Alternatively, during a pandemic with high mortality rates, incentives for reporting symptoms might be present, such as access to scarce antiviral medications and medical care. In both situations, a comprehensive screening approach may be necessary, which uses ITDS for fever screening and a health questionnaire to detect other symptoms or exposures that would increase specificity of the screening process. Finally, the usefulness of any infectious disease screening must take into account temperature fluctuations, use of antipyretic medications, transmission risks, prevalence of infections, and asymptomatic infections.
This study had several limitations. Measurement error resulting from variation in digital oral thermometer measurement and technique may have decreased the correlation between ITDS and oral temperature measurements (38). For FLIR and Wahl, varying readouts by different operators may have led to increased variability. This method, although necessary for direct temperature comparisons, may have decreased the accuracy of FLIR and Wahl. Use of alarm features as recommended by the manufacturers could minimize these differences but might lead to more false-positive results. In addition, unlike the other 2 devices, Wahl required calibration to ambient temperature once per day, but room temperatures varied within the day. We evaluated only systems submitted by manufacturers to the bid process, thus limiting the generalizability of our results to other devices.
To assess the sensitivity and specificity of different ITDS for fever detection and to determine their optimal thresholds, we validated each measurement by oral thermometry, which required a clinical setting. Thus, generalizability to settings such as airports and border crossings may be limited. Substantial delays to travelers and ethical concerns such as follow-up treatment made it impractical to conduct this study in an airport setting. In addition, although a few studies have examined screenings in airports, they confirmed temperature only in febrile persons, thus sensitivity and specificity of ITDS could not be established from such studies.
The sensitivity and specificity of screening by using ITDS are determined by the selected fever temperature cutoff, which tends to be 2–3 degrees lower than the standard fever threshold because of differences between skin and core temperatures. Increasing or decreasing sensitivity causes a reciprocal change in specificity. For example, lowering OptoTherm’s threshold from the optimal 95.7°F to 94.5°F would achieve almost 100% sensitivity but would reduce specificity to 63.6% and increase the false-positive rate to 36.4%; to reach near 100% specificity with the OptoTherm by using a cutoff of 100°F for ITDS, sensitivity decreases to 6.4%.
Maximizing accuracy by choosing the optimal cutoff with the highest sensitivity and specificity may not be practical in a real-world setting, considering the relative costs of false-positive and false-negative results. In settings where secondary evaluation is available or during a pandemic with high illness severity, ITDS temperature can be set at a lower cutoff to ensure fewer false negatives, each of which represents a potential public health threat. However, setting the cutoff to achieve very high sensitivity can result in many false positives, which could have adverse consequences to the population being screened (e.g., unnecessary travel delays, missed work) and increase the workload of staff who are conducting the screening. In settings where confirmatory testing may not be feasible or high costs may be associated with a false-positive result, a higher ITDS temperature cutoff may be preferable.
Ms Nguyen is a Council of State and Territorial Epidemiologists fellow in applied epidemiology at the Centers for Disease Control and Prevention, Atlanta. Her research interests focus on the epidemiology and surveillance of infectious diseases, particularly in travelers.
Acknowledgments
We thank the emergency department staff of Grady Memorial Hospital, John H. Stroger, Jr. Hospital of Cook County, and Presbyterian Healthcare Services for collaborating with CDC; Shannon Bachar, Sena Blumensaadt, Heather Hastings, Jane Keir, Krista Kornylo, Lisa Poray, Efrosini Roland, Michelle Russell, and Evelyn Chris Swager for contributions to data collection; Daniel Rodriguez for translation of study forms; Francisco Averhoff and Peter Houck for guidance during protocol development; and Nabiha Megateli-Das for editorial contributions.
This article is dedicated to Harvey Lipman, an outstanding scientist and colleague whose substantial contributions to public health research will always be remembered.
References
- Murphy FA, Nathanson N. The emergence of new virus diseases: an overview. Semin Virol. 1994;5:87–102. DOIGoogle Scholar
- St John RK, King A, de Jong D, Bodie-Collins M, Squires SG, Tam TW. Border screening for SARS. Emerg Infect Dis. 2005;11:6–10.PubMedGoogle Scholar
- Bell DM. Public health interventions and SARS spread, 2003. Emerg Infect Dis. 2004;10:1900–6.PubMedGoogle Scholar
- Institute of Medicine. Quarantine stations at ports of entry protecting the public’s health. Washington: National Academy Press; 2005.
- Pang X, Zhu Z, Xu F, Guo J, Gong X, Liu D, Evaluation of control measures implemented in the severe acute respiratory syndrome outbreak in Beijing, 2003. JAMA. 2003;290:3215–21. DOIPubMedGoogle Scholar
- Teo P, Yeoh BS, Ong SN. SARS in Singapore: surveillance strategies in a globalising city. Health Policy. 2005;72:279–91. DOIPubMedGoogle Scholar
- Wang LM, Chen YC, Tung SP, Chen CY, Chang SC, Chiang SC, The rationale of fever surveillance to identify patients with severe acute respiratory syndrome in Taiwan. Emerg Med J. 2006;23:202–5. DOIPubMedGoogle Scholar
- Cowling BJ, Lau LL, Wu P, Wong HW, Fang VJ, Riley S, Entry screening to delay local transmission of 2009 pandemic influenza A (H1N1). BMC Infect Dis. 2010;10:82. DOIPubMedGoogle Scholar
- Ferguson NM, Cummings DA, Cauchemez S, Fraser C, Riley S, Meeyai A, Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature. 2005;437:209–14. DOIPubMedGoogle Scholar
- Malone JD, Brigantic R, Muller GA, Gadgil A, Delp W, McMahon BH, U.S. airport screening in response to pandemic influenza: modeling and analysis. Travel Med Infect Dis. 2009;7:181–91. DOIPubMedGoogle Scholar
- Tham KY. An emergency department response to severe acute respiratory syndrome: a prototype response to bioterrorism. Ann Emerg Med. 2004;43:6–14. DOIPubMedGoogle Scholar
- Lee CW, Tsai YS, Wong TW, Lau CC. A loophole in international quarantine procedures disclosed during the SARS crisis. Travel Med Infect Dis. 2006;4:22–8. DOIPubMedGoogle Scholar
- Han K, Zhu X, He F, Liu L, Zhang L, Ma H, Lack of airborne transmission during outbreak of pandemic (H1N1) 2009 among tour group members, China, June 2009. Emerg Infect Dis. 2009;15:1578–81.PubMedGoogle Scholar
- Monto AS, Gravenstein S, Elliott M, Colopy M, Schweinle J. Clinical signs and symptoms predicting influenza infection. Arch Intern Med. 2000;160:3243–7. DOIPubMedGoogle Scholar
- Public Health Agency of Canada. Thermal image scanners to detect fever in airline passengers, Vancouver and Toronto, 2003. Can Commun Dis Rep. 2004;30:165–7.PubMedGoogle Scholar
- Chiu WT, Lin PW, Chiou HY, Lee WS, Lee CN, Yang YY, Infrared thermography to mass-screen suspected SARS patients with fever. Asia Pac J Public Health. 2005;17:26–8. DOIPubMedGoogle Scholar
- Chen WK, Wu HD, Lin CC, Cheng YC. Emergency department response to SARS, Taiwan. Emerg Infect Dis. 2005;11:1067–73.PubMedGoogle Scholar
- Rothman RE, Hsieh YH, Yang S. Communicable respiratory threats in the ED: tuberculosis, influenza, SARS, and other aerosolized infections. Emerg Med Clin North Am. 2006;24:989–1017. DOIPubMedGoogle Scholar
- Bitar D, Goubar A, Desenclos JC. International travels and fever screening during epidemics: a literature review on the effectiveness and potential use of non-contact infrared thermometers. Euro Surveill. 2009;4:19115.PubMedGoogle Scholar
- Ng EYK, Kaw GJ, Chang WM. Analysis of IR thermal imager for mass blind fever screening. Microvasc Res. 2004;68:104–9. DOIPubMedGoogle Scholar
- Liu CC, Chang RE, Chang WC. Limitations of forehead infrared body temperature detection for fever screening for severe acute respiratory syndrome. Infect Control Hosp Epidemiol. 2004;25:1109–11. DOIPubMedGoogle Scholar
- Chan LS, Cheung GT, Lauder IJ, Kumana CR. Screening for fever by remote-sensing infrared thermographic camera. J Travel Med. 2004;11:273–9. DOIPubMedGoogle Scholar
- Hausfater P, Zhao Y, Defrenne S, Bonnet P, Riou B. Cutaneous infrared thermometry for detecting febrile patients. Emerg Infect Dis. 2008;14:1255–8. DOIPubMedGoogle Scholar
- Ng DK, Chan CH, Lee RS, Leung LC. Non-contact infrared thermometry temperature measurement for screening fever in children. Ann Trop Paediatr. 2005;25:267–75. DOIPubMedGoogle Scholar
- Chiang MF, Lin PW, Lin LF, Chiou HY, Chien CW, Chu SF, Mass screening of suspected febrile patients with remote-sensing infrared thermography: alarm temperature and optimal distance. J Formos Med Assoc. 2008;107:937–44. DOIPubMedGoogle Scholar
- Smith LS. Reexamining age, race, site, and thermometer type as variables affecting temperature measurement in adults—a comparison study. BMC Nurs. 2003;2:1. DOIPubMedGoogle Scholar
- Weinert D. Circadian temperature variation and ageing. Ageing Res Rev. 2010;9:51–60.DOIPubMedGoogle Scholar
- Mackowiak PA, Wasserman SS, Levine MM. A critical appraisal of 98.6 degrees F, the upper limit of the normal body temperature, and other legacies of Carl Reinhold August Wunderlich. JAMA. 1992;268:1578–80. DOIPubMedGoogle Scholar
- Ng EYK, Acharya RU. Remote-sensing infrared thermography. IEEE Eng Med Biol Mag. 2009;28:76–83. DOIPubMedGoogle Scholar
- Ivanitsky GR, Khizhnyak EP, Deev AA, Khizhnyak LN. Thermal imaging in medicine: a comparative study of infrared systems operating in wavelength ranges of 3–5 and 8–12 microm as applied to diagnosis. Dokl Biochem Biophys. 2006;407:59–63. DOIPubMedGoogle Scholar
- Sund-Levander M, Grodzinsky E. What is the evidence base for the assessment and evaluation of body temperature? Nurs Times. 2010;106:10–3.PubMedGoogle Scholar
- O’Grady NP, Barie PS, Bartlett JG, Bleck T, Carroll K, Kalil AC, Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med. 2008;36:1330–49. DOIPubMedGoogle Scholar
- Hooker EA, Houston H. Screening for fever in an adult emergency department: oral vs tympanic thermometry. South Med J. 1996;89:230–4.PubMedGoogle Scholar
- Dodd SR, Lancaster GA, Craig JV, Smyth RL, Williamson PR. In a systematic review, infrared ear thermometry for fever diagnosis in children finds poor sensitivity. J Clin Epidemiol. 2006;59:354–7. DOIPubMedGoogle Scholar
- Hooper VD, Andrews JO. Accuracy of noninvasive core temperature measurement in acutely ill adults: the state of the science. Biol Res Nurs. 2006;8:24–34. DOIPubMedGoogle Scholar
- Samaan G, Patel M, Spencer J, Roberts L. Border screening for SARS in Australia: what has been learnt? Med J Aust. 2004;180:220–3.PubMedGoogle Scholar
- Webby R, Krause V. Evaluation of SARS audit at Darwin International Airport. Northern Territory Disease Control Bulletin. 2003;10:8–10.
- Jensen BN, Jensen FS, Madsen SN, Lossl K. Accuracy of digital tympanic, oral, axillary, and rectal thermometers compared with standard rectal mercury thermometers. Eur J Surg. 2000;166:848–51. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 16, Number 11—November 2010
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Nicole J. Cohen, Centers for Disease Control and Prevention, 1600 Clifton Rd NE, Mailstop E03, Atlanta, GA 30333, USA
Top