Volume 17, Number 12—December 2011
Dispatch
Hepatitis E Virus Antibodies in Blood Donors, France
Cite This Article
Citation for Media
Abstract
Using a validated sensitive assay, we found hepatitis E virus (HEV) IgG in 52.5% of voluntary blood donors in southwestern France. This finding suggests HEV is highly endemic to this region. The high HEV prevalence may reflect local dietary practices, such as eating uncooked pork and game products.
It is now recognized that hepatitis E virus infection is not confined to developing countries. HEV infection is a growing public health concern in industrialized countries where the disease is mainly autochthonous, caused by HEV genotypes 3 (Europe) and 4 (People’s Republic of China and Japan), and is thought to be zoonotic (1).
In a previous study, we estimated that 16.6% of blood donors in the Midi-Pyrénées region of southwestern France have HEV antibodies (2). This rate was much higher than that measured in northern France (3), which suggests differences between these 2 populations and their exposure to HEV that we wished to explore further. However, it is difficult to make wider comparisons with seroprevalence studies from other areas because the various assays used differed in sensitivity and specificity (4). Because a recent study suggested that the HEV IgG assay we used in our original study lacks sensitivity (5), we repeated and extended the study using a more sensitive assay that has been validated by using serum from PCR-proven HEV genotype 3 infections (5).
During September 2003 through May 2004, serum samples were collected from 512 adult blood donors 18–64 years old (median 42 years) and 188 children 2–4 years old. The blood donors were unpaid voluntary donors; the children were hospitalized in Toulouse for surgery or trauma. All were residents of the Midi-Pyrénées region. The prevalence of HEV IgG was determined by using the Wantai HEV IgG enzyme immunoassay (Wantai Biologic Pharmacy Enterprise, Beijing, People’s Republic of China), according to the manufacturer’s instructions. Details of baseline demographic data and putative risk factors were collected from blood donors by using a structured questionnaire. In addition, to assess the risk for foodborne infection, we tested 18 local pig-liver sausages for HEV RNA using a quantitative real-time PCR based on the open reading frame 2 region of the HEV genome (6).
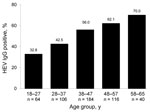
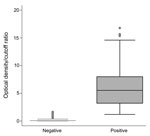
HEV IgG was detected in 268 (52.5%) of 512 (95% confidence interval [CI] 48.2%–56.8%) of the blood donors. Seroprevalence increased with age (Figure 1). The ranges of optical density/cutoff ratios for positive and negative samples showed a clear bimodal distribution (Figure 2). Of 244 rural donors, 63.1% (95% CI 57%–69.2%) were anti-HEV positive compared with 42.9% (95% CI 37–48.8) of 268 urban donors (p<0.01). For children, seroprevalence was 3.7% (95% CI 1.0%–6.5%). The mean ± SD optical density/cutoff ratio of the positive samples was 5.43 ± 3.93 for children and 5.99 ± 3.52 for adults. Although several factors were associated with the presence of HEV IgG after univariate analysis, multivariate analysis identified only age, rural residence, hunting, and contact with cats as factors independently associated with HEV IgG positivity (Table 1).
HEV RNA was found in 8 (44%) of the 18 sausages tested by real-time PCR (Table 2). The virus load ranged from 100 (the limit of detection for this assay) to 668,520 copies/g. We attempted to genotype HEV RNA–positive samples by sequencing a 189-nt fragment of the open reading frame 2 gene (7). This was successful only for the sample with the highest virus load. The virus was identified as HEV genotype 3.
We determined that the HEV IgG prevalence among blood donors in Midi-Pyrénées is 52.5%, the highest seroprevalence reported in an industrialized country. This rate is 3.1 times higher than our previous estimate (16.6%) for the same population (2). The implication is that HEV is hyperendemic to Midi-Pyrénées.
Although surprising, we believe these results are valid for several reasons. First, the Wantai assay used to assess HEV seroprevalence has been validated for this purpose in the United Kingdom, another region where HEV-3 predominates (5). The greater proportion of reactive serum seen with this assay is unlikely to have resulted from nonspecific reactivity because the assay produced a clear distinction between negative and positive samples, and only a small proportion of young children tested positive. Our findings agree with those of another study that found a much increased HEV seroprevalence when the more accurate test was used (5). Second, autochthonous HEV genotype 3 hepatitis in Midi-Pyrénées is common. The estimated rate of acquisition of HEV infection in organ transplant recipients in Toulouse is 3.2 per 100 person-years (8). This figure is derived from regular monitoring by using sensitive molecular techniques and does not depend on serologic assays. As noted in other countries (9,10), the percentage of HEV-positive serum increased with age, which is consistent with cumulative exposure to infection over time.
HEV is usually transmitted orally, and foodborne transmission of zoonotic strains has been demonstrated. Hunting of wild boar and deer is popular in Midi-Pyrénées, particularly in rural areas. Both species have been identified as sources of human infection (11). The consumption of uncooked game meat, which is traditional in this area, could explain the high HEV antibody prevalence in 20 (80%) of 25 hunters. Further evidence comes from a case–control study among organ transplant recipients in Midi-Pyrénées that demonstrated that the only factor independently associated with HEV infection was consumption of game meat (6). Some of these foods have been shown to contain HEV RNA, and phylogenetic analysis demonstrated that these strains were closely related to human strains (8).
Another suspected zoonotic source of HEV genotype 3 infection is the domestic pig (12). Hepatitis E cases have been linked to eating uncooked pork-liver sausage in southeastern France (13). We found that a high proportion (44%) of pig-liver sausages purchased in Toulouse contained HEV RNA. These air-dried sausages are popular in Midi-Pyrénées and are usually eaten raw. Their infectivity is unknown, but cell-culture experiments have demonstrated that high virus loads correlate with high infectivity (14).
In addition to direct foodborne transmission, the growing boar population in Midi-Pyrénées and the spreading of pig manure on land may pose indirect risks through fecal contamination of soil and watercourses. These 2 factors and the 2 foodborne sources of HEV might explain the high HEV antibody prevalence in Midi-Pyrénées and the statistical association of HEV seropositivity with rural residence and hunting, but they cannot explain the association with cat contact. HEV RNA has not yet been detected in domestic cats.
The high percentage of donors with HEV antibodies in our area contrasts with the low recorded incidence of autochthonous hepatitis E in France and other industrialized countries (1,15). Even though we documented dozens of hepatitis E cases in Midi-Pyrénées during the past decade, the remarkably high seroprevalence in this area suggests that most infections must be subclinical or unrecognized. However, in susceptible persons, such as organ transplant recipients and patients with chronic liver disease, the consequences of HEV infection are grave and raise public health, as well as food and environmental safety, concerns.
HEV is highly endemic to the Midi-Pyrénées region in southwestern France. We showed that seroprevalence increases with age and is associated with rural residence, hunting, and exposure to cats. These associations became apparent only when we used a sensitive assay to detect HEV IgG. The reasons for the high HEV prevalence in this population are uncertain but may be due, at least partially, to the culinary culture of the local community. Thorough cooking of game meat and pork products would help minimize the risk for HEV infection and could form part of a public health initiative in this area.
Dr Mansuy is a physician in the virology laboratory at the Toulouse University Hospital. His research interests include hepatitis E, enteric, and respiratory viruses.
Acknowledgments
We thank Wantai Biological Pharmacy Enterprise, Beijing, China, for providing the anti-HEV IgG kits at no cost.
The Royal College of Physicians (London) supporting this work by the award of the Dame Sheila Sherlock Travelling Fellowship. Agence Nationale de la Recherche France, grant PNRA HEVZOONEPI, provided financial support on HEV research.
References
- Dalton HR, Bendall R, Ijaz S, Banks M. Hepatitis E: an emerging infection in developed countries. Lancet Infect Dis. 2008;8:698–709. DOIPubMedGoogle Scholar
- Mansuy JM, Legrand-Abravanel F, Calot JP, Peron JM, Alric L, Agudo S, High prevalence of anti-hepatitis E virus antibodies in blood donors from south west France. J Med Virol. 2008;80:289–93. DOIPubMedGoogle Scholar
- Boutrouille A, Bakkali-Kassimi L, Cruciere C, Pavio N. Prevalence of anti-hepatitis E virus antibodies in French blood donors. J Clin Microbiol. 2007;45:2009–10. DOIPubMedGoogle Scholar
- Herremans M, Bakker J, Duizer E, Vennema H, Koopmans MP. Use of serological assays for diagnosis of hepatitis E virus genotype 1 and 3 infections in a setting of low endemicity. Clin Vaccine Immunol. 2007;14:562–8. DOIPubMedGoogle Scholar
- Bendall R, Ellis V, Ijaz S, Ali R, Dalton H. A comparison of two commercially available anti-HEV IgG kits and a re-evaluation of anti-HEV IgG seroprevalence data in developed countries. J Med Virol. 2010;82:799–805. DOIPubMedGoogle Scholar
- Legrand-Abravanel F, Kamar N, Sandres-Saune K, Garrouste C, Dubois M, Mansuy JM, Characteristics of autochthonous hepatitis E virus infection in solid-organ transplant recipients in France. J Infect Dis. 2010;202:835–44. DOIPubMedGoogle Scholar
- Legrand-Abravanel F, Mansuy JM, Dubois M, Kamar N, Peron JM, Rostaing L, Hepatitis E virus genotype 3 diversity, France. Emerg Infect Dis. 2009;15:110–4. DOIPubMedGoogle Scholar
- Legrand-Abravanel F, Kamar N, Sandres-Saune K, Lhomme S, Mansuy JM, Muscari F, Hepatitis E virus infection without reactivation in solid-organ transplant recipients, France. Emerg Infect Dis. 2011;17:30–7. DOIPubMedGoogle Scholar
- Dalton HR, Stableforth W, Thurairajah P, Hazeldine S, Remnarace R, Usama W, Autochthonous hepatitis E in southwest England: natural history, complications and seasonal variation, and hepatitis E virus IgG seroprevalence in blood donors, the elderly and patients with chronic liver disease. Eur J Gastroenterol Hepatol. 2008;20:784–90. DOIPubMedGoogle Scholar
- Bouwknegt M, Engel B, Herremans MM, Widdowson MA, Worm HC, Koopmans MP, Bayesian estimation of hepatitis E virus seroprevalence for populations with different exposure levels to swine in the Netherlands. Epidemiol Infect. 2008;136:567–76. DOIPubMedGoogle Scholar
- Takahashi K, Kitajima N, Abe N, Mishiro S. Complete or near-complete nucleotide sequences of hepatitis E virus genome recovered from a wild boar, a deer, and four patients who ate the deer. Virology. 2004;330:501–5. DOIPubMedGoogle Scholar
- Meng XJ. From barnyard to food table: the omnipresence of hepatitis E virus and risk for zoonotic infection and food safety. Virus Res. 2011;161:23–30. DOIPubMedGoogle Scholar
- Colson P, Borentain P, Queyriaux B, Kaba M, Moal V, Gallian P, Pig liver sausage as a source of hepatitis E virus transmission to humans. J Infect Dis. 2010;202:825–34. DOIPubMedGoogle Scholar
- Okamoto H. Efficient cell culture systems for hepatitis E virus strains in feces and circulating blood. Rev Med Virol. 2011;21:18–31. DOIPubMedGoogle Scholar
- Renou C, Moreau X, Pariente A, Cadranel JF, Maringe E, Morin T, A national survey of acute hepatitis E in France. Aliment Pharmacol Ther. 2008;27:1086–93. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 17, Number 12—December 2011
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Jacques Izopet, INSERM U1043, Laboratoire de Virologie, Hôpital Purpan, CHU Toulouse, Toulouse, F31300 France
Top