Volume 31, Number 4—April 2025
Research Letter
Rabbit Hepatitis E Virus, Ukraine, 2024
Cite This Article
Citation for Media
Abstract
We identified hepatitis E virus (HEV) 3ra, a potentially zoonotic subtype, in rabbits in Ukraine, which highlights their potential role as reservoirs. Screening of rabbit fecal samples identified HEV-3ra. Public health services in this country should improve HEV surveillance and expand HEV sampling.
Hepatitis E virus (HEV), family Hepeviridae, species Paslahepevirus balayani, is a major cause of acute enterically transmitted hepatitis worldwide, particularly in developing nations, where its public health impact is substantial (1). P. balayani has 8 genotypes (2). Zoonotic genotypes 3 and 4 are globally distributed and transmitted to humans through consumption of infected animal products or contact with infected animals, particularly pigs (3). Rabbits (Oryctolagus cuniculus) have been identified as reservoirs for HEV, specifically the potentially zoonotic HEV-3ra subtype linked to human infections, with cases reported during 2015–2016 (4). Despite widespread detection of HEV in Western and Central Europe, its prevalence and the role of animals in transmission remain underexplored in Eastern Europe, especially Ukraine. That knowledge gap is concerning because of the zoonotic risk from rabbit meat consumption or environmental exposure (5). The risk might be heightened in situations of armed conflict, where greater reliance on bushmeat could increase the chances of spillover events. We investigated the prevalence and molecular characterization of HEV in domestic rabbits from Ukraine.
In April 2024, we collected 108 fresh fecal samples from clinically healthy Californian domestic rabbits (O. cuniculus) born and raised on private family farms in the Sumy region, northeastern Ukraine. We suspended samples in 10% phosphate-buffered saline (pH 7.2) and centrifuged at 8,000 × g for 5 minutes. We extracted total nucleic acids from 200 µL of the supernatant by using the QIAamp Cador Pathogen Mini Kit (QIAGEN, https://www.qiagen.com) on a QIAcube automated system, according to the manufacturer’s protocol. For HEV RNA, we used a pangenotypic quantitative reverse transcription PCR (RT-PCR) targeting the conserved open reading frame (ORF) 3 region (6), then used a broad-spectrum nested RT-PCR targeting the ORF1 region (7). We used the World Health Organization PEI 6329/10 subgenotype 3a (GenBank accession no. AB630970) as a positive control. We performed real-time PCR on a CFX96 Real-Time PCR System (Bio-Rad Laboratories, https://www.bio-rad.com) by using the NZYSupreme One-Step RT-qPCR Probe Master Mix (2×) kit plus ROX dye (NZYTech, https://www.nzytech.com). We performed nested RT-PCR on a T100 thermocycler (Bio-Rad Laboratories) by using the Xpert One-Step RT-PCR Kit (GriSP, https://grisp.pt) in the first round and the Xpert Fast Hotstart Mastermix 2× with dye (GriSP) in the second round, according to the manufacturer’s instructions. We used Sanger sequencing to sequence the suspected positive amplicons in both directions. We aligned trimmed sequences by using Sequence Alignment Editor version 7.1.9 (BioEdit, https://bioedit.software.informer.com) and compared with nucleotide sequences from GenBank.
To further analyze positive samples, we used sequence-independent single-primer amplification with a previously described protocol (8), and the sequence-independent single-primer amplification samples underwent next-generation sequencing at Novogene (https://www.novogene.com) by using the NovaSeq 6000 platform (Illumina, https://www.illumina.com). We trimmed data by using BBduk version 38.86 (https://jgi.doe.gov/data-and-tools/software-tools/bbtools) to remove adaptor sequences and low-quality bases. We mapped the trimmed reads to the HEV reference genome by using BBMap version 38.86 (https://jgi.doe.gov/data-and-tools/software-tools/bbtools), processed the reads with Samtools version 1.18 (http://www.htslib.org) to generate binary alignment map files, and created a consensus FASTA sequence. We conducted phylogenetic analysis by using MEGA X software version 10.2 (https://www.megasoftware.net) and applied the maximum-likelihood method with 1,000 bootstrap replicates. We used the HEVnet tool (https://www.rivm.nl/en/hevnet) for further genotyping (9).
We detected HEV RNA in 1 (0.93%, 95% CI 0.02%–5.05%) rabbit stool sample from the 108 samples tested. We obtained 1 genome fragment from ORF2 and 2 fragments from ORF1 (Table). Sequence analysis confirmed the genome identity as HEV. The ORF2 fragment (GenBank accession no. PQ541216) showed identity with 2 human sequences from Switzerland: 92.04% identity with GenBank accession no. OX044324 and 91.72% with GenBank accession no. OV844765. One ORF1 fragment (GenBank accession no. PQ541214) showed identity with rabbit sequences from Australia: 91.42% with GenBank accession no. MW002522 and 90.99% with GenBank accession no. MZ676756. The other ORF1 fragment (GenBank accession no. PQ541215) displayed lower similarity to sequences from human samples from France (87.59% with GenBank accession no. MF444074) and rabbit samples from China (86.57% with GenBank accession no. KX227751).
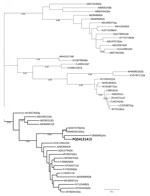
Phylogenetic analysis confirmed clustering of the obtained RNA-dependent RNA polymerase genome fragment to the rabbit-associated HEV-3ra (Figure). The HEV-3ra prevalence in rabbits reported in this study (0.93%) is lower than in another study from Europe, in which rabbit-associated HEV prevalence ranged from 7% to 23% in domestic and wild rabbits (5). However, the absence of HEV RNA has also been reported in other regions of Europe, such as in Portugal (10). Those differences could be attributed to regional variations in sampling and detection methods, environmental factors, and surveillance intensity.
In summary, this study provides evidence for HEV circulation in Ukraine, specifically the potentially zoonotic HEV-3ra in a domestic rabbit, addressing a key gap in its epidemiology. The genetic similarity of the detected HEV-3ra strain to those found in humans and animals elsewhere highlights the subgenotype’s zoonotic potential and risk for cross-border transmission. Future research should expand sampling across species and regions using molecular and serologic studies to clarify transmission dynamics and public health risks. The findings in this study emphasize the need for robust surveillance in animals and humans to clarify HEV circulation.
Mr. Santos-Silva is a PhD student at the School of Medicine and Biomedical Sciences, Porto, Portugal. His research interests focus on the molecular detection, epidemiology, and cross-species transmission of zoonotic pathogens, with a particular emphasis on hepatitis E virus and other emerging infectious diseases in domestic and wild animal reservoirs.
Acknowledgments
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The PhD work of S.S.-S. was supported by the Fundação para a Ciência e a Tecnologia (scholarship 2021.09461.BD contract through the Maria de Sousa-2021 program). A.R.-J. was supported by a contract from the Spanish Junta de Andalucía (Nicolas Monardes program: C1-0001-2023).
All authors contributed to the study’s conception and design, commented on previous versions of the manuscript, and read and approved the final manuscript. Material preparation, data collection, and analysis were performed by S.S.-S. and J.R.M. The first draft of the manuscript was written by S.S.-S. and J.R.M.
References
- World Health Organization. Hepatitis E [cited 2024 Dec 25]. https://www.who.int/news-room/fact-sheets/detail/hepatitis-e.
- Smith DB, Izopet J, Nicot F, Simmonds P, Jameel S, Meng XJ, et al. Update: proposed reference sequences for subtypes of hepatitis E virus (species Orthohepevirus A). J Gen Virol. 2020;101:692–8. DOIPubMedGoogle Scholar
- Velavan TP, Pallerla SR, Johne R, Todt D, Steinmann E, Schemmerer M, et al. Hepatitis E: An update on One Health and clinical medicine. Liver Int. 2021;41:1462–73. DOIPubMedGoogle Scholar
- Abravanel F, Lhomme S, El Costa H, Schvartz B, Peron JM, Kamar N, et al. Rabbit hepatitis E virus infections in humans, France. Emerg Infect Dis. 2017;23:1191–3. DOIPubMedGoogle Scholar
- Lhomme S, Dubois M, Abravanel F, Top S, Bertagnoli S, Guerin JL, et al. Risk of zoonotic transmission of HEV from rabbits. J Clin Virol. 2013;58:357–62. DOIPubMedGoogle Scholar
- Frías M, López-López P, Zafra I, Caballero-Gómez J, Machuca I, Camacho Á, et al. Development and clinical validation of a pangenotypic PCR-based assay for the detection and quantification of hepatitis E virus (Orthohepevirus A genus). J Clin Microbiol. 2021;59:1–7. DOIPubMedGoogle Scholar
- Johne R, Plenge-Bönig A, Hess M, Ulrich RG, Reetz J, Schielke A. Detection of a novel hepatitis E-like virus in faeces of wild rats using a nested broad-spectrum RT-PCR. J Gen Virol. 2010;91:750–8. DOIPubMedGoogle Scholar
- Moreno G, O’connor D. Sequence-independent, single-primer amplification of RNA viruses V.2 [cited 2024 July 15]. DOIGoogle Scholar
- Mulder AC, Kroneman A, Franz E, Vennema H, Tulen AD, Takkinen J, et al.; Members of HEVnet. HEVnet: a One Health, collaborative, interdisciplinary network and sequence data repository for enhanced hepatitis E virus molecular typing, characterisation and epidemiological investigations. Euro Surveill. 2019;24:1–6. DOIPubMedGoogle Scholar
- Santos-Silva S, Santos N, López-López P, Nascimento MSJ, Gonçalves HMR, Van der Poel WHM, et al. Hepatitis E virus in wild and domestic rabbits from Portugal: a combined molecular and longitudinal serological study. Vet Res Commun. 2024;48:3283–9. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: March 17, 2025
Table of Contents – Volume 31, Number 4—April 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
João R. Mesquita, University of Porto, Rua Jorge Viterbo Ferreira nr. 228, Porto 4050-313, Portugal
Top