Volume 17, Number 7—July 2011
CME ACTIVITY - Synopsis
Neurognathostomiasis, a Neglected Parasitosis of the Central Nervous System
Cite This Article
Citation for Media
Introduction

Medscape, LLC is pleased to provide online continuing medical education (CME) for this journal article, allowing clinicians the opportunity to earn CME credit.
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and Emerging Infectious Diseases. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit(s)TM. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test and/or complete the evaluation at www.medscape.org/journal/eid; (4) view/print certificate.
Release date: June 27, 2011; Expiration date: June 27, 2012
Learning Objectives
Upon completion of this activity, participants will be able to:
- Describe food sources of gnathostomiasis parasitic infection
- Describe different clinical manifestations of neurognathostomiasis infection
- Identify methods of diagnosis of neurognathostomiasis
- Describe treatment options for gnathostomiasis
- Describe outcomes of gnathostomiasis infection
Medscape CME Editor
Nancy Mannikko, Technical Writer/Editor, Emerging Infectious Diseases. Disclosure: Nancy Mannikko has disclosed no relevant financial relationships.
Medscape CME Author
Desiree Lie, MD, MSEd, Clinical Professor; Director of Research and Faculty Development, Department of Family Medicine, University of California Irvine at Orange. Disclosure: Désirée Lie, MD, MSEd, has disclosed the following relevant financial relationship: served as a nonproduct speaker for "Topics in Health" for Merck Speaker Services.
Authors
Disclosures: Juri Katchanov, MD; Kittisak Sawanyawisuth, MD; Verajit Chotmongkol, MD; and Yukifumi Nawa, MD, have disclosed no relevant financial relationships.
Abstract
Gnathostomiasis is a foodborne zoonotic helminthic infection caused by the third-stage larvae of Gnathostoma spp. nematodes. The most severe manifestation involves infection of the central nervous system, neurognathostomiasis. Although gnathostomiasis is endemic to Asia and Latin America, almost all neurognathostomiasis cases are reported from Thailand. Despite high rates of illness and death, neurognathostomiasis has received less attention than the more common cutaneous form of gnathostomiasis, possibly because of the apparent geographic confinement of the neurologic infection to 1 country. Recently, however, the disease has been reported in returned travelers in Europe. We reviewed the English-language literature on neurognathostomiasis and analyzed epidemiology and geographic distribution, mode of central nervous system invasion, pathophysiology, clinical features, neuroimaging data, and treatment options. On the basis of epidemiologic data, clinical signs, neuroimaging, and laboratory findings, we propose diagnostic criteria for neurognathostomiasis.
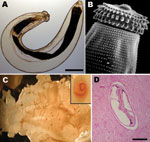
Foodborne parasitic infections are common in the tropics, where many foodborne parasites are endemic and ingestion of raw shellfish and freshwater fish, as well as undercooked meat, is frequent among local populations (1). Increased international travel to areas endemic for these foodborne parasites and migration from tropical areas have led to the emergence of these diseases in temperate climates (2), where such infections are rarely seen by physicians and thus may not be considered in differential diagnoses. Gnathostomiasis is a foodborne zoonotic helminthic infection caused by the third-stage larva of Gnathostoma spp. nematodes (Figure 1, panels A, B). At least 13 species have been identified (3), with 5 recorded in humans. G. spinigerum is the most common of these nematodes in Asia. Human infection with G. hispidum, G. doloresi, and G. nipponicum were found only in Japan (4). In the Americas, G. binucleatum is the only proven pathogenic Gnathostoma nematode in humans. Humans are infected primarily by eating raw or undercooked freshwater fish (Figure 1, panel C), frogs, and chicken. Humans are accidental unsuitable hosts; the parasite rarely develops to an adult worm, and the disease in humans is caused by the migrating larva.
Gnathostomiasis can be divided into cutaneous, visceral, and ocular forms, depending on the site of larval migration and subsequent signs and symptoms (2). The most common clinical presentation is the cutaneous one (Figure 1, panel D), which is characterized by localized, intermittent, migratory swellings of the skin and is often associated with localized pain, pruritus, and erythema (5,6). Visceral involvement can manifest in virtually any organ and any part of the body (3). The most severe manifestation of the visceral disease is involvement of the central nervous system (CNS), i.e., neurognathostomiasis. Neurognathostomiasis has been reported only in G. spinigerum infections (3).
We found 24 reports describing a total of 248 patients with neurognathostomiasis published in English-language literature. In this article, we review epidemiology, mode of CNS invasion, pathophysiology, clinical features, neuroimaging data, and treatment options, and we propose diagnostic criteria for this emerging disease.
The first clinical case of G. spinigerum meningoencephalitis was reported by Daensvang in 1949 (7). Eighteen years later, Chitanondh and Rosen (8) documented the first pathologic evidence of G. spinigerum invasion of the CNS from the autopsy of a 34-year-old Thai woman. In their seminal study, Boongird et al. (9) delineated the clinical syndromes of neurognathostomiasis, which facilitated further prospective and retrospective case identification (10). With the advance of high-resolution neuroimaging, the parasitic tracks of Gnathostoma could be demonstrated on magnetic resonance imaging (MRI), aiding the diagnosis of neurognathostomiasis in the absence of worm recovery (11).
Most (241/248) identified cases of neurognathostomiasis reported in the English-language literature were from Thailand. Two patients were infected in Laos (12,13), 1 in Japan (14), 1 in Myanmar (13), and 2 in South Korea (15,16). One patient had traveled to Southeast Asia and Japan (17), so the geographic origin of his infection could not be determined. The incubation period of neurognathostomiasis can be as long as 10 years because of the long persistence of the nematode larvae in the soft tissues before CNS invasion (2,15), which means it can occur in immigrants in a country in which the parasite is not endemic (16,18). All case-patients were reported to be infected with G. spinigerum. Although gnathostomiasis has been increasingly detected outside of Asia, particularly in Mexico, no cases of neurognathostomiasis have been reported in other regions. The occurrence of another Gnathostoma species in Latin America, namely G. binucleatum, is a possible explanation for this phenomenon.
The first case of neurognathostomiasis in a traveler was reported in 2003 (19). Since then, 4 patients in Europe returning from gnathostomiasis-endemic countries have been published (13,20,21). Two patients traveled to Thailand (19,20), 1 patient to Lao People’s Democratic Republic (13) and 1 patient to Myanmar (13). The patients were reported to have indulged in local customs of eating raw fish (19) and shrimp (20). Gnathostomiasis has been recently reported in travelers returning from southern Africa (22,23). However, the patients had cutaneous manifestation of Gnathostoma spp. infection without neurologic involvement.
Gnathostoma spp. nematodes are highly invasive parasites. After being ingested, the third-stage larvae penetrate the mucosal wall of the gastrointestinal tract. Larvae can enter the CNS by invading directly through the loose connective tissues of the neural foramina of the skull base along the cranial nerves and vessels or through intervertebral foramina along the spinal nerves and vessels (24). The larvae release a variety of molecules into their surrounding environment that facilitate tissue penetration and invasion (25). Proteases are the major molecular types among various components of these so-called excretor–secretory products, both quantitatively and qualitatively (26). In Gnathostoma spp., matrix metalloproteinases might play a key role in invasion of host tissues (27).
Gnathostoma spp. nematodes typically enter the spinal cord along the nerve roots, causing radiculomyelitis (9,28). The parasite is then able to ascend the spinal cord and reach the brain (3,29). This journey may take as long as several years and has been documented in a Thai patient through sequential imaging (29).
Given the length of the migrating larva, which averages 3.0 mm (30), the migration of the worm in the CNS causes direct mechanical injury because of tearing of nerve tissue. The hallmark sign of neurognathostomiasis is the evidence of multiple hemorrhagic tracks; these tracks have been documented throughout the spinal cord and brain tissue by postmortem examination (9), as well as through neuroimaging (31). Subarachnoid hemorrhage can result from larvae burrowing through a cerebral arteriole (32,33). Eosinophilic meningitis caused by Gnathostoma spp. is characterized by erythrocytes in cerebrospinal fluid (CSF) (33), which suggests mechanical damage by the worm. Apart from the structural damage, the inflammatory response to the migrating larvae might further contribute to tissue destruction (34).
The main clinical syndromes of neurognathostomiasis are radiculomyelitis/ radiculomyeloencephalitis, meningitis/meningoencephalitis, and subarachnoid and intracerebral hemorrhage (Table 1). Each syndrome reflects the tissue injury by the migrating parasite. Because the larvae migrate, the same patient can show sequentially different neurologic syndromes. For example, in 1 Laotian patient, meningomyelitis developed, followed by subarachnoid hemorrhage (12). Meningitis, subdural hemorrhage, and then intraparenchymal lesions developed sequentially in 1 patient from northeastern Thailand (29). The most common symptom reported virtually by every patient was pain: radicular pain in cases of spinal involvement (often lasting 1–5 days) or headache in cases of meningoencephalitis. The most common manifestation, spinal cord disease, comprised 55% of all clinical syndromes (Table 1).
Myelopathy in gnathostomiasis is characterized by radicular pain followed by an ascending paresis of lower extremities or quadriparesis with bladder dysfunction (9,10,33,35–38). A sensory level for all modalities including vibration and proprioception was a frequent finding, commonly localized in the thoracic region (9). Cerebral and meningeal involvement is characterized by meningeal signs and decreased consciousness. Abducens nerve palsy was the most common cranial nerve impairment (9,33). Intracranial hemorrhages were seen with sudden-onset focal neurologic deficits such as hemiparesis and hemihypaesthesia (9,10,18,33).
Isolated Neurologic Disease
The most common clinical feature of gnathostomiasis is intermittent migratory cutaneous swelling (5). However, in the largest published series of neurognathostomiasis in 162 patients, only 11 (7%) had a history of cutaneous lesions (10). Unilateral eyelid swelling, another typical sign of gnathostomiasis, occurred in only 6 patients (4%) (10). Boongird et al. reported only 3 patients with subcutaneous swelling out of 24 patients with neurognathostomiasis; cutaneous gnathostomiasis developed in all of these patients after the onset of neurologic symptoms (9).
CSF Studies
Eosinophilic pleocytosis is a hallmark laboratory finding. CSF eosinophilia in patients is usually prominent; median values were 40% (n = 24) and 54% (n = 39) in the studies by Boongird et al (9) and Schmutzhard et al (33), respectively. In 109 (67%) of patients reported by Punyagupta et al. (10), the eosinophil count in CSF was >30%. CSF has been reported as xanthochromic or bloody in 134 patients (64% of all published cases). CSF glucose is usually only mildly reduced but was reported as low as 11 mg/dL (9% of the plasma value) in 1 patient (37).
Neuroradiologic Features
Neuroimaging of 11 patients with neurognathostomiasis was reported in English-language literature. On cranial computed tomography, subarachnoid hemorrhage and intracerebral hemorrhage were the most common findings (12,18,33). In 1 patient a subdural hemorrhage was detected (29). The hallmark of cerebral gnathostomiasis on MRI was the detection of hemorrhagic tracks (Figure 2, panels A, C, D). The magnetic resonance signal of a hemorrhagic lesion varies in acute, subacute, and chronic lesions. Hence, on T1 and T2 weighted imaging both hypointensities and hyperintensities have been documented. No gradient-echo T2-weighted sequences have been reported in neurognathostomiasis. However, gradient-echo T2-weighted sequences are exquisitely sensitive to local field inhomogeneities of hematoma and could become a useful sequence in radiologic assessment of cerebral gnathostomiasis.
Diffuse multisegmental spinal cord swelling with corresponding T2 hyperintensities was the most common finding on spinal imaging (11,17) (Figure 2, panel B). Gadolinium enhancement of the lesions was reported in cranial and spinal MRI of 4 patients as slight, nodular, and ill-defined (11,17,35). Table 2 summarizes typical findings on neuroimaging in neurognathostomiasis.
Immunodiagnosis
The detection of antibodies in serum is the main pillar of immunodiagnosis of gnathostomiasis. Two methods have been established for clinical routine: ELISA and Western blot by using crude Gnathostoma spp. antigens from the larval extract. The 24-kD band on Western blot was shown to have nearly 100% specificity for gnathostomiasis (39). The current practice for serologic diagnosis is to use the ELISA (e.g., multiple-dot ELISA) as the first step (40) and to confirm the results by Western blot. At least 2 patients seroconverted during their neurologic illness (19,20), which emphasizes the need for repeated serologic examination in cases with strong clinical suspicion. The serologic testing can be done in several laboratories, including those in Thailand (Bangkok, Khon Kaen), Japan (Miayzaki), and Europe (Basel, Switzerland). Additional information about laboratories providing this service is available from the corresponding author.
Differential Diagnosis
The main differential diagnosis of neurognathostomiasis is an infection with Angiostrongylus cantonensis. However, Angiostrongylus spp. infection is usually seen as self-limited eosinophilic meningitis and only rarely causes severe disease with prominent spinal or cerebral involvement (1). Immunodiagnostically, positive 24-kD band against G. spinigerum antigen and negative 29-kD and 31-kD bands on immunoblot against A. cantonensis antigen make a diagnosis of an Angiostrongylus spp. infection highly unlikely (40). Other causes of a parasitic CNS disease, such as cysticercosis, toxocariasis, schistosomiasis, baylisascariasis, or paragonimiasis, can be distinguished by epidemiologic distribution, clinical signs and symptoms, radiologic appearance, and serologic testing.
No randomized trials of antihelminthic therapy have been conducted for neurognathostomiasis. We have identified 9 reports of treatment of neurognathostomiasis with albendazole (11,13,17–21,29); 5 patients fully recovered, 2 partially recovered, and 2 patients did not recover. Outcomes might reflect the natural course of the disease rather than the effect of treatment. In several reports, antihelminthic treatment was considered as potentially detrimental and was withheld on the basis of its possible induction of brain edema due to sudden helminthic death (31,38). Three returned travelers from Thailand were treated with albendazole (800 mg/d for 1 month [19], 800 mg/d for 3 weeks [20], and 400 mg 2×/d for 4 weeks [21]) and fully recovered (19–21). In all these patients, dexamethasone (oral or intravenous) or predinsolone was added to the treatment regimen. A recent retrospective evaluation of long-term treatment efficacy in 13 patients from France with imported gnathostomiasis reported 2 patients with neurologic involvement (13), 1 with myelitis, and 1 with encephalitis (C. Strady, pers. comm.). The patient with myelitis fully recovered after albendazole therapy, and the patient with encephalitis was reported to have had 4 relapses despite therapy with ivermectin and albendazole (13; C. Strady, pers. comm.).
Corticosteroids have been used in neurognathostomiasis to treat cerebral and spinal edema (19–21). They also might prevent or alleviate paradoxical worsening after initiation of antihelminthic treatment. However, no conclusion about their efficacy can be drawn from the current data because no randomized control trials have been conducted and the retrospective data are too limited to allow any comparison between the groups. At the moment, no evidence-based recommendations can be issued.
Outcome
The case-fatality rate in the first series of 24 patients was 25% (9). Later studies reported mortality rates of 7%–12% (10,33). However, as the authors remark, these case-fatality rates are almost certainly an underestimate because many patients in critical condition were discharged home to die (10). In total, the outcome of 247 patients has been reported in the literature. An unfavorable outcome, e.g., death or severe persistent disability, was reported in 78 patients (32%). However, the recent case reports indicate that the current prognosis might be better. Of 15 patients in whom neurognathostomiasis was diagnosed after 2001, poor outcomes were reported in 3 (20%) patients. In all 4 clinical cases of returned travelers with myelitis, recovery was good after antihelminthic therapy (13,19–21).
Definitive diagnosis of helminthic CNS infections is notoriously difficult. The recovery rate of worms from the CSF is low, and the invasive procedures for diagnosis are seldom justified (33,35). Neurognathostomiasis is no exception; of 248 published cases, only 27 (11%) were confirmed by recovery of larvae. Moreover, an intermittent subcutaneous swelling, the hallmark of gnathostomiasis, is found only in the minority of neurognathostomiasis cases. Given these difficulties, clinical criteria are useful for practice and research. On the basis of the analysis of published cases, we propose diagnostic criteria that should guide a clinician if a pathologic diagnosis is not possible (Table 3).
Neurognathostomiasis is a parasitic CNS disease endemic to Southeast Asia, particularly Thailand. Because of growing international travel to disease-endemic areas, as well as migration from the tropics, an increasing number of patients will seek medical attention in Europe and North America. Neurognathostomiasis should be suspected in patients who have eosinophilic radiculomyelitis, myeloencephalitis or meningoencephalitis, and a history of travel to gnathostomiasis-endemic areas. Ingestion of raw or inadequately cooked shellfish, freshwater fish, or poultry is a prerequisite for infection; however, because such information might not be volunteered by the patient, clinicians should ask directly about foods eaten during travel. Serologic testing, including Western blot, shows high specificity and can contribute to the diagnosis in an appropriate clinical and epidemiologic setting. No evidence-based protocol is available for treatment of neurognathostomiasis; however, a 3- to 4-week course of albendazole with prednisolone or dexamethasone is 1 of the most frequently used regimens. Clinicians should advise patients traveling to or living in disease-endemic areas to avoid eating undercooked freshwater fish, frogs, poultry, and shellfish.
Dr Katchanov is a neurologist and a member of the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand. He has a special interest in neuroinfectious disorders, particularly bacterial meningitis, and neurohelminthiases.
References
- Nawa Y, Hatz C, Blum J. Sushi delights and parasites: the risk of fishborne and foodborne parasitic zoonoses in Asia. Clin Infect Dis. 2005;41:1297–303. DOIPubMedGoogle Scholar
- Moore DA, McCroddan J, Dekumyoy P, Chiodini PL. Gnathostomiasis: an emerging imported disease. Emerg Infect Dis. 2003;9:647–50.PubMedGoogle Scholar
- Nawa Y. Historical review and current status of gnathostomiasis in Asia. Southeast Asian J Trop Med Public Health. 1991;22(Suppl):217–9.PubMedGoogle Scholar
- Herman JS, Chiodini PL. Gnathostomiasis, another emerging imported disease. Clin Microbiol Rev. 2009;22:484–92. DOIPubMedGoogle Scholar
- Rusnak JM, Lucey DR. Clinical gnathostomiasis: case report and review of the English-language literature. Clin Infect Dis. 1993;16:33–50. DOIPubMedGoogle Scholar
- Chai JY, Han ET, Shin EH, Park JH, Chu JP, Hirota M, An outbreak of gnathostomiasis among Korean emigrants in Myanmar. Am J Trop Med Hyg. 2003;69:67–73.PubMedGoogle Scholar
- Daengsvang S. Human gnathostomiasis in Siam with reference to the method of prevention. J Parasitol. 1949;35:116–21. DOIPubMedGoogle Scholar
- Chitanondh H, Rosen L. Fatal eosinophilic encephalomyelitis caused by the nematode Gnathostoma spinigerum. Am J Trop Med Hyg. 1967;16:638–45.PubMedGoogle Scholar
- Boongird P, Phuapradit P, Siridej N, Chirachariyavej T, Chuahirun S, Vejjajiva A. Neurological manifestations of gnathostomiasis. J Neurol Sci. 1977;31:279–91. DOIPubMedGoogle Scholar
- Punyagupta S, Bunnag T, Juttijudata P. Eosinophilic meningitis in Thailand. Clinical and epidemiological characteristics of 162 patients with myeloencephalitis probably caused by Gnathostoma spinigerum. J Neurol Sci. 1990;96:241–56. DOIPubMedGoogle Scholar
- Sawanyawisuth K, Tiamkao S, Kanpittaya J, Dekumyoy P, Jitpimolmard S. MR imaging findings in cerebrospinal gnathostomiasis. AJNR Am J Neuroradiol. 2004;25:446–9.PubMedGoogle Scholar
- Brant-Zawadzki M, Wofsy CB, Schechter G. CT-evidence of subarachnoid hemorrhage due to presumed gnathostomiasis. West J Med. 1982;137:65–7.PubMedGoogle Scholar
- Strady C, Dekumyoy P, Clement-Rigolet M, Danis M, Bricaire F, Caumes E. Long-term follow-up of imported gnathostomiasis shows frequent treatment failure. Am J Trop Med Hyg. 2009;80:33–5.PubMedGoogle Scholar
- Kawamura J, Kohri Y. Nobuyuki 0. Eosinophilic meningoradiculo-myelitis caused by Gnathostoma spinigerum: a case report. Arch Neurol. 1983;40:583–5.PubMedGoogle Scholar
- Lo Re V III, Gluckman SJ. Eosinophilic meningitis due to Gnathostoma spinigerum. J Infect. 2002;45:117–20. DOIPubMedGoogle Scholar
- Catalano M, Kaswan D, Levi MH. Wider range for parasites that cause eosinophilic meningitis. Clin Infect Dis. 2009;49:1283. DOIPubMedGoogle Scholar
- Chandenier J, Husson J, Canaple S, Gondry-Jouet C, Dekumyoy P, Danis M, Medullary gnathostomiasis in a white patient: use of immunodiagnosis and magnetic resonance imaging. Clin Infect Dis. 2001;32:E154–7. DOIPubMedGoogle Scholar
- Germann R, Schächtele M, Nessler G, Seitz U, Kniehl E. Cerebral gnathostomiasis as a cause of an extended intracranial bleeding. Klin Padiatr. 2003;215:223–5. DOIPubMedGoogle Scholar
- Górgolas M, Santos-O'Connor F, Unzú AL, Fernández-Guerrero ML, Gárate T, Troyas Guarch RM, Cutaneous and medullar gnathostomiasis in travelers to Mexico and Thailand. J Travel Med. 2003;10:358–61. DOIPubMedGoogle Scholar
- Elzi L, Decker M, Battegay M, Rutishauser J, Blum J. Chest pain after travel to the tropics. Lancet. 2004;363:1198. DOIPubMedGoogle Scholar
- Schmutzhard E. Eosinophilic myelitis, a souvenir from South East Asia. Pract Neurol. 2007;7:48–51.PubMedGoogle Scholar
- Herman JS, Wall EC, van-Tulleken C, Godfrey-Faussett P, Bailey RL, Chiodini PL. Gnathostomiasis acquired by British tourists in Botswana. Emerg Infect Dis. 2009;15:594–7. DOIPubMedGoogle Scholar
- Hale DC, Blumberg L, Frean J. Case report: gnathostomiasis in two travelers to Zambia. Am J Trop Med Hyg. 2003;68:707–9.PubMedGoogle Scholar
- Katchanov J, Nawa Y. Helminthic invasion of the central nervous system: many roads lead to Rome. Parasitol Int. 2010;59:491–6. DOIPubMedGoogle Scholar
- Maruyama H, Nawa Y. Immunology of the infection. In: Murrell KD, Fried B, editors. Worldclass parasites, vol. 11, food-borne parasitic zoonoses. Fish and plant-borne parasites. New York: Springer; 2007. p. 337–81.
- Tort J, Brindley PJ, Knox D, Wolfe KH, Dalton JP. Proteinases and associated genes of parasitic helminths. Adv Parasitol. 1999;43:161–266. DOIPubMedGoogle Scholar
- Uparanukraw P, Morakote N, Harnnoi T, Dantrakool A. Molecular cloning of a gene encoding matrix metalloproteinase-like protein from Gnathostoma spinigerum. Parasitol Res. 2001;87:751–7. DOIPubMedGoogle Scholar
- Punyagupta S, Limtrakul C, Vichipanthu P, Karnchanachetanee C, Nye SW. Radiculomyeloencephalitis associated with eosinophilic pleocytosis—report of nine cases. Am J Trop Med Hyg. 1968;17:551–60.PubMedGoogle Scholar
- Sawanyawisuth K, Chlebicki MP, Pratt E, Kanpittaya J, Intapan PM. Sequential imaging studies of cerebral gnathostomiasis with subdural hemorrhage as its complication. Trans R Soc Trop Med Hyg. 2009;103:102–4. DOIPubMedGoogle Scholar
- Rojekittikhun W, Pubampen S. Morphological variation and abnormality of cephalic hooklets of Gnathostoma spinigerum hepatic stage larvae from laboratory infected mice. Southeast Asian J Trop Med Public Health. 1998;29:118–22.PubMedGoogle Scholar
- Sithinamsuwan P, Chairangsaris P. Images in clinical medicine. Gnathostomiasis—neuroimaging of larval migration. N Engl J Med. 2005;353:188. DOIPubMedGoogle Scholar
- Visudhiphan P, Chiemchanya S, Somburanasin R, Dheandhanoo D. Causes of spontaneous subarachnoid hemorrhage in Thai infants and children. A study of 56 patients. J Neurosurg. 1980;53:185–7. DOIPubMedGoogle Scholar
- Schmutzhard E, Boongird P, Vejjajiva A. Eosinophilic meningitis and radiculomyelitis in Thailand, caused by CNS invasion of Gnathostoma spinigerum and Angiostrongylus cantonensis. J Neurol Neurosurg Psychiatry. 1988;51:80–7. DOIPubMedGoogle Scholar
- Punyagupta S, Juttijudata P, Bunnag T, Comer DS. Two fatal cases of eosinophilic myeloencephalitis a newly recognized disease caused by Gnathostoma spinigerum. Trans R Soc Trop Med Hyg. 1968;62:801–9. DOIPubMedGoogle Scholar
- Bunyaratavej K, Pongpunlert W, Jongwutiwes S, Likitnukul S. Spinal gnathostomiasis resembling an intrinsic cord tumor/myelitis in a 4-year-old boy. Southeast Asian J Trop Med Public Health. 2008;39:800–3.PubMedGoogle Scholar
- Bunnag T, Comer DS, Punyagupta S. Eosinophilic myeloencephalitis caused by Gnathostoma spinigerum. Neuropathology of nine cases. J Neurol Sci. 1970;10:419–34. DOIPubMedGoogle Scholar
- Daengsvang S. A monograph on the genus Gnasthostoma and gnathostomiasis in Thailand. Tokyo: Southeast Asian Medical Information Center International Medical Foundation of Japan; 1980.
- Sawanyawisuth K, Tiamkao S, Nitinavakarn B, Dekumyoy P, Jitpimolmard S. MR imaging findings in cauda equina gnathostomiasis. AJNR Am J Neuroradiol. 2005;26:39–42.PubMedGoogle Scholar
- Laummaunwai P, Sawanyawisuth K, Intapan PM, Chotmongkol V, Wongkham C, Maleewong W. Evaluation of human IgG class and subclass antibodies to a 24 kDa antigenic component of Gnathostoma spinigerum for the serodiagnosis of gnathostomiasis. Parasitol Res. 2007;101:703–8. DOIPubMedGoogle Scholar
- Eamsobhana P, Ongrotchanakun J, Yookek A, Punthuprapasa P, Monkong N, Dekumyoy P. Multi-immunodot for rapid differential diagnosis of eosinophilic meningitis due to parasitic infections. J Helminthol. 2006;80:249–54.PubMedGoogle Scholar
Figures
Tables
Follow Up
Earning Medscape CME Credit
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions and earn continuing medical education (CME) credit, please go to www.medscape.org/journal/eid. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the New Users: Free Registration link on the left hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, CME@medscape.net. For technical assistance, contact CME@webmd.net. American Medical Association's Physician's Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/category/2922.html. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit is acceptable as evidence of participation in CME activities. If you are not licensed in the US and want to obtain an AMA PRA CME credit, please complete the questions online, print the certificate and present it to your national medical association.
Neurognathostomiasis, a Neglected Parasitosis of the Central Nervous System
Medscape CME Questions
1. A traveler returning from Thailand is suspected of having neurognathostomiasis. Which of the following is least likely to be a food source for the infection?
A. Undercooked pork
B. Undercooked chicken
C. Raw fish
D. Undercooked frog
2. In the patient from question 1, which of the following is likely to be the most common presentation?
A. Intracerebral hemorrhage with headache and hemiparesis
B. Myelopathy with radicular pain and ascending paresis
C. Meningoencephalitis with photophobia and seizures
D. Subarachnoid hemorrhage with loss of consciousness
3. Which of the following is considered the most accurate test for the diagnosis of neurognathostomiasis?
A. Cerebrospinal fluid microscopy
B. Brain biopsy
C. Western blot serology
D. Spinal fluid culture
4. Which of the following treatments are believed to be most efficacious based on clinical trials?
A. Albendazole alone for 4 weeks
B. Albendazole with prednisolone
C. Ivermectin with albendazole
D. None of the above
5. Which of the following best describes the most recent reports (after 2000) of rates of poor outcomes associated with gnathostomiasis infection?
A. Over 50% mortality
B. Mortality or severe disability rate of 40%
C. Poor outcome in 30%
D. Poor outcome in 20%
Activity Evaluation
| 1. The activity supported the learning objectives. | ||||
| Strongly Disagree |
Strongly Agree
|
|||
|
1
|
2
|
3
|
4
|
5
|
| 2. The material was organized clearly for learning to occur. | ||||
| Strongly Disagree |
Strongly Agree
|
|||
|
1
|
2
|
3
|
4
|
5
|
| 3. The content learned from this activity will impact my practice. | ||||
| Strongly Disagree |
Strongly Agree
|
|||
|
1
|
2
|
3
|
4
|
5
|
| 4. The activity was presented objectively and free of commercial bias. | ||||
| Strongly Disagree |
Strongly Agree
|
|||
|
1
|
2
|
3
|
4
|
5
|
Related Links
Table of Contents – Volume 17, Number 7—July 2011
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Yukifumi Nawa, Faculty of Tropical Medicine, Mahidol University, 420/6 Rajavithi Rd, Bangkok 10400, Thailand
Top