Volume 17, Number 8—August 2011
Research
Seroprevalence of Trichodysplasia Spinulosa–associated Polyomavirus
Figure 1
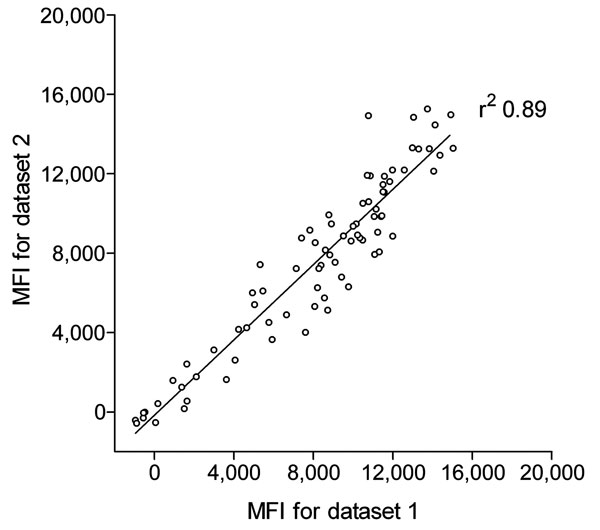
Figure 1. Reproducibility of trichodysplasia spinulosa–polyomavirus (TSV) viral protein 1 (VP1) immunoassay. Seroreactivity against TSV VP1 in 80 renal transplant patients, the Netherlands, was analyzed twice by using the Bio-Plex 100 analyzer (Bio-Rad Laboratories, Hercules, CA, USA). Datasets 1 and 2 were obtained during a 3-month interval by using freshly coupled identical glutathione–casein bead sets coupled independently with the same crude TSV VP1 bacterial extract. Each circle represents 1 serum sample, and the line represents results of linear regression analyses. Correlation coefficient (r2) was determined by using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA, USA). MFI, median fluorescent intensity.
Page created: August 15, 2011
Page updated: August 15, 2011
Page reviewed: August 15, 2011
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.