Volume 18, Number 7—July 2012
Dispatch
Influenza Virus Infection in Guinea Pigs Raised as Livestock, Ecuador
Cite This Article
Citation for Media
Abstract
To determine whether guinea pigs are infected with influenza virus in nature, we conducted a serologic study in domestic guinea pigs in Ecuador. Detection of antibodies against influenza A and B raises the question about the role of guinea pigs in the ecology and epidemiology of influenza virus in the region.
Influenza A virus infection causes disease in humans and domestic animals, including pigs, horses, and chickens, and seasonal epidemics among humans in the Northern and Southern Hemispheres result in hospitalizations and deaths worldwide. Influenza A virus transmission studies are often conducted in laboratory guinea pigs (1,2) because the virus can efficiently spread from infected animals to naive guinea pigs by direct and indirect (short-range infectious aerosols) contact (3,4). However, whether guinea pigs are naturally infected with influenza virus outside the laboratory setting is not known.
In some regions of South America, guinea pigs are part of the traditional cuisine and are produced as livestock and sold commercially for human consumption. Guinea pigs are customarily raised on small rural farms in proximity to other livestock. Circulation of influenza virus in these populations has not been studied. Given the effect of influenza virus on human health and the susceptibility of guinea pigs to influenza virus infection in the laboratory, it is worthwhile to determine whether influenza virus can spread among guinea pigs in agricultural settings. As an initial step in this endeavor, we obtained serum samples from domestic guinea pigs in Ecuador and tested them for the presence of influenza antibodies to determine whether the guinea pigs had been infected with influenza virus.
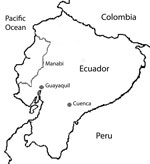
We obtained serum samples from 40 guinea pigs from 3 different regions of Ecuador (Figure 1), 20 from Cuenca and 10 each from Guayaquil and the Manabí region. Cuenca is located in the Andes region, 2,500 m above sea level; it is one of the main producers of guinea pig meat. Guayaquil, the most populated city in Ecuador, is located at the head of the Gulf of Guayaquil on the Pacific Ocean. The Manabí region is located in western Ecuador on the Pacific Ocean coast. Serum samples were collected from adult guinea pigs that we purchased from local farms (Cuenca), where they had been raised as livestock, or from live animal markets (Guayaquil and Manabí). The samples were collected by heart puncture under general anesthesia (combination of ketamine and xylazine). Animals were euthanized after samples were obtained.
For antigens in the serologic analyses, we used whole viruses and recombinant hemagglutinin, nucleoprotein, and neuraminidase (subtypes N1 and N2) proteins produced in our laboratory as described (5,6). The whole viruses were A/Brisbane/59/2007 (H1N1) (Brisbane07), A/New Caledonia/20/1999 (H1N1) (Newcal99), A/Wisconsin/67/2005 (H3N2) (Wisconsin05), A/Vietnam/1203/2004 (H5N1) (Vietnam04), and B/Yamagata/16/1988 (Yamagata88). The hemagglutinins were A/California/04/2009 (Cal09), NewCal99, Vietnam04, Wisconsin05, and Yamagata88. The nucleoproteins were Brisbane07, A/Puerto Rico/08/1934, and B/Florida/04/2006. The subtype N1 neuraminidases were Cal09 and Vietnam04; the N2 subtype was A/Hong Kong/1/1968.
ELISA was done as described (7), with slight modifications for the use of guinea pig serum. The cutoff for serum considered positive for influenza virus was the value of the negative control (naive guinea pig serum) +3 SD (from 3 repetitions). For Western blot (WB) analyses (8), serum samples were pooled into groups of 5 (groups 1–4, 5–6, and 7–8 were from Cuenca, Guayaquil, and Manabí region, respectively). Pooled samples were also used for the hemagglutination inhibition (HI) assay, as described (9), using influenza strains Brisbane07, Wisconsin05, and Vietnam04. As positive controls, we used serum from guinea pigs that we had infected with Cal09, Brisbane07, NewCal99, Wisconsin05, Vietnam04, or Yamagata88.
ELISA results for the 40 serum samples showed that 20, 18, and 14 were positive for influenza subtypes H1, H3, and H5, respectively (Table 1). The samples were also tested for the presence of antibodies against the influenza virus nucleoprotein: results for 29 were positive. Samples with positive results to >1 hemagglutinin antigens and to nucleoprotein were also analyzed for the presence of antibodies against proteins of the neuraminidase subtypes N1 (Cal09, Vietnam04) and N2 (influenza A/Hong Kong/1/1968 [H3N2]) (Table 1). Serum samples were also tested for the presence of influenza B virus by using whole virus, recombinant hemagglutinin, and recombinant nucleoprotein as described above. Samples from Cuenca showed the highest overall positivity to the 3 antigens (Table 2).
WB analysis of pooled samples from the 3 areas confirmed the ELISA results that showed antibodies against the hemagglutinin and nucleoprotein antigens; however, samples from Manabí region (7,8) showed immunoreactivity to the H3 antigen only (Figure 2). WB results for serum samples from Cuenca and Guayaquil were positive for influenza B virus, but results were not positive for samples from Manabí when whole virus was used as antigen (Figure 2). HI activity was observed in all pooled samples against all 3 viruses tested. Titers were highest in samples from Cuenca (320 for Brisbane07, 640 for Wisconsin05, and 80 for Vietnam04) and lowest for samples from Manabí (80 for Brisbane07 and 40 for Wisconsin05 and Vietnam04). Titers for samples from Guayaquil were 160 for Brisbane07 and 80 for Wisconsin05 and Vietnam04.
We showed the presence of influenza virus antibodies with HI activity to different virus subtypes in guinea pigs marketed through local farms and live animal markets in different regions of Ecuador. Seroprevalence was similar for influenza A virus subtypes H1 (50%) and H3 (45%). A study of influenza among humans in Ecuador also showed similar seroprevalences for subtypes H1 (5.1%) and H3 (5.5%) (10). This10-fold difference between seroprevalence rates among humans and the guinea pigs in our study may be explained by animal husbandry practices that facilitate influenza transmission (e.g., the crowding of animals in cages).
The presence of antibodies to influenza B virus in guinea pigs is notable because infection with this virus is thought to be restricted to humans (11). Recent studies in our laboratory demonstrated that guinea pigs can also be infected with influenza B viruses and that they readily transmit the virus to naive guinea pigs (12). These studies support our finding that influenza B virus has the potential to be transmitted from humans to other species. Further studies are needed to isolate and characterize the type B influenza virus present in the population of guinea pigs to determine if there has been an adaptation to the new host or if the guinea pig is only a transient reservoir for the human virus.
We found that several guinea pigs had antibodies against influenza A (H5). Ecuador is purported to be free of avian influenza (13); however, reports from other countries in South America demonstrate the presence of avian subtypes H3, H5N2, H7N3, and H13N9 in wild birds (14). In addition, chickens, turkeys, and guinea pigs that are later sold in local farmers’ markets are raised together by some families, thereby facilitating contact of guinea pigs with avian influenza viruses. We tested only for seroreactivity to the H5 hemagglutinin and the N1 neuraminidase; therefore, further studies are needed to determine whether different avian origin influenza viruses are present in the guinea pig population.
We did not determine whether guinea pigs are an incidental host for influenza virus infection or, if instead, the virus has been adapted to these animals or if guinea pigs are a natural reservoir for some influenza viruses. To this end, virus isolation and characterization would be necessary to determine the virus strains circulating in this population. In the laboratory, guinea pigs are infected and efficiently transmit influenza viruses to naive hosts without showing any overt clinical signs of disease (1). Therefore, further studies are needed to address the specific role of guinea pigs raised as livestock in the ecology and epidemiology of influenza viruses in the region.
Dr. Leyva-Grado is a postdoctoral fellow at Mount Sinai School of Medicine in New York. His primary research interests include the study of the pathophysiology of virus-induced respiratory infections in humans and domestic animals and the development of therapeutics for virus infections.
Acknowledgment
This work was partially funded by the Center of Excellence for Influenza Research and Surveillance, National Institutes of Health (grant HHSN266200700010C to P.P.) and the Keck Foundation (P.P.). F.K. was supported by an Erwin Schrödinger fellowship (J 3232) from the Austrian Science Fund.
References
- Lowen AC, Mubareka S, Tumpey TM, García-Sastre A, Palese P. The guinea pig as a transmission model for human influenza viruses. Proc Natl Acad Sci U S A. 2006;103:9988–92. DOIPubMedGoogle Scholar
- Sun Y, Bi Y, Pu J, Hu Y, Wang J, Gao H, Guinea pig model for evaluating the potential public health risk of swine and avian influenza viruses. PLoS ONE. 2010;5:e15537. DOIPubMedGoogle Scholar
- Mubareka S, Lowen AC, Steel J, Coates AL, García-Sastre A, Palese P. Transmission of influenza virus via aerosols and fomites in the guinea pig model. J Infect Dis. 2009;199:858–65. DOIPubMedGoogle Scholar
- Lowen AC, Steel J, Mubareka S, Palese P. High temperature (30°C) blocks aerosol but not contact transmission of influenza virus. J Virol. 2008;82:5650–2. DOIPubMedGoogle Scholar
- Krammer F, Schinko T, Palmberger D, Tauer C, Messner P, Grabherr R. Trichoplusia ni cells (High FiveTM) are highly efficient for the production of influenza A virus–like particles: a comparison of two insect cell lines as production platforms for influenza vaccines. Mol Biotechnol. 2010;45:226–34. DOIPubMedGoogle Scholar
- Pica N, Hai R, Krammer F, Wang TT, Maamary J, Eggink D, Hemagglutinin stalk antibodies elicited by the 2009 pandemic influenza virus as a mechanism for the extinction of seasonal H1N1 viruses. Proc Natl Acad Sci U S A. 2012;109:2573–8. DOIPubMedGoogle Scholar
- Wang TT, Tan GS, Hai R, Pica N, Ngai L, Ekiert DC, Vaccination with a synthetic peptide from the influenza virus hemagglutinin provides protection against distinct viral subtypes. Proc Natl Acad Sci U S A. 2010;107:18979–84. DOIPubMedGoogle Scholar
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4. DOIPubMedGoogle Scholar
- Lowen AC, Steel J, Mubareka S, Carnero E, García-Sastre A, Palese P. Blocking interhost transmission of influenza virus by vaccination in the guinea pig model. J Virol. 2009;83:2803–18. DOIPubMedGoogle Scholar
- Douce RW, Aleman W, Chicaiza-Ayala W, Madrid C, Sovero M, Delgado F, Sentinel surveillance of influenza-like illness in two cities of the tropical country Ecuador: 2006–2010. PLoS ONE. 2011;6:e22206. DOIPubMedGoogle Scholar
- Jackson D, Elderfield RA, Barclay WS. Molecular studies of influenza B virus in the reverse genetics era. J Gen Virol. 2011;92:1–17. DOIPubMedGoogle Scholar
- Pica N, Chou YY, Bouvier NM, Palese P. Transmission of influenza B viruses in the guinea pig. J Virol. 2012;86:4279–87. DOIPubMedGoogle Scholar
- Panamerican Health Organization. Plan nacional de contingencia para enfrentar posible pandemia de influenza en el Ecuador. 2005 [cited 2011 Oct 11]. http://new.paho.org/hq/dmdocuments/2010/NIPP_ecuador_2005.pdf
- Senne DA. Avian influenza in North and South America, the Caribbean, and Australia, 2006–2008. Avian Dis. 2010;54:179–86. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 18, Number 7—July 2012
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Peter Palese, Mount Sinai School of Medicine,1 Gustave Levy Place, New York, NY 10029-6574, USA
Top