Volume 18, Number 7—July 2012
Research
Loss of Household Protection from Use of Insecticide-Treated Nets against Pyrethroid-Resistant Mosquitoes, Benin
Cite This Article
Citation for Media
Abstract
Pyrethroid resistance is becoming widespread in Anopheles gambiae mosquitoes, coinciding with expanded use of insecticide-treated nets (ITNs) throughout Africa. To investigate whether nets in use are still protective, we conducted household trials in northern and southern Benin, where An. gambiae mosquitoes are susceptible and resistant, respectively, to pyrethroids. Rooms were fitted with window traps and monitored for mosquito biting and survival rates before and after the nets were treated with pyrethroid. Sleeping under an ITN in the location with resistant mosquitoes was no more protective than sleeping under an untreated net, regardless of its physical condition. By contrast, sleeping under an ITN in the location with susceptible mosquitoes decreased the odds of biting by 66%. ITNs provide little or no protection once the mosquitoes become resistant and the netting acquires holes. Resistance seriously threatens malaria control strategies based on ITN.
Insecticide-treated nets (ITNs) and long-lasting insecticidal nets (LLINs) are the primary interventions for preventing malaria in sub-Saharan Africa (1,2). Nets accumulate holes through wear and tear during the course of everyday use, but the pyrethroid treatment continues to provide personal protection and to reduce vector capacity through excito-repellency and the killing of mosquitoes that contact the net (3,4). During the last decade, pyrethroid resistance in Anopheles gambiae mosquitoes became widespread in western Africa and spread to or developed in eastern Africa (5–9). As coverage of LLINs expands across the continent under programs supported by the President’s Malaria Initiative and Global Fund (10), resistance will inevitably increase (11–13).
Although resistance is perceived as a serious threat to the future of malaria control, the current distribution of resistance is patchy, and its severity seems to differ from 1 location to another. In the western African country of Benin, pyrethroid resistance has evolved in the M (Mopti) molecular form of An. gambiae mosquitoes that appears to combine the knockdown resistance (kdr) gene with oxidase mechanisms (14,15). Carriers of this resistance were not controlled by pyrethroid treatments in experimental hut trials of ITNs or the leading brands of LLINs, PermaNet 2.0 (Vestergaard Frandsen SA, Aarhus, Denmark) and Olyset (Sumitomo Chemicals, Osaka, Japan) (16,17). However, further west in Côte d’Ivoire, the kdr in An. gambiae S (Savannah) form mosquitoes conferred only limited resistance, and trials of ITNs continued to protect against mosquito blood feeding (biting) and malaria transmission by this species (18–20).
Results from experimental hut trials in Benin raise an alarm. Of key concern is whether ITNs that are subject to wear and tear under everyday household conditions fail to protect ITN users now that An. gambiae mosquitoes are becoming resistant. Modern mosquito nets lack physical durability, and household nets can accrue an average of 12–20 holes during 1–2 years of use (21). Net replacement schemes struggle to meet demand at this level of deterioration and attrition. To assess protection conferred by in-use polyester nets, we compared nets in households of northern Benin, where An. gambiae mosquitoes are mostly susceptible to pyrethroids, with nets in households of southern Benin, where An. gambiae mosquitoes are mostly resistant (7,16,22). Residents of the selected households were all regular users of nets.
Study Sites
Three suburbs (Ladji, Fifadji, and Abomey Calavi) of Cotonou in southern Benin support breeding of mosquitoes of An. gambiae M form that is mostly pyrethroid resistant with a high frequency of kdr (>90%) (14,22). Malanville, 800 km north of Cotonou, is situated in an area in which mosquitoes are mainly pyrethroid susceptible, where An. gambiae M form mosquitoes show a kdr frequency of <0.05 (7,14).
Selection of Households and Torn Nets
We selected 3–5 households from each site. The criteria for selection were that each house contain a sleeping room with a close-fitting door and a window suitable for fitting a mosquito exit trap and in which occupants possessed >1 worn nets under regular use. The points of entry for mosquitoes were through open doors or eave gaps between walls and roofs. Nets were made of polyester, cotton, or nylon and contained holes of various sizes and number. Before inclusion, the nets were subjected to World Health Organization cone bioassays by using a laboratory-susceptible strain of An. gambiae to detect pyrethroid residue. Only untreated nets or nets that had lost their insecticide through washing were retained for the study.
Household members gave informed consent to participate in the study and were provided with chemoprophylaxis throughout. The London School of Hygiene & Tropical Medicine and the Benin national ethics committees granted ethics approval.
Mosquito Exit Window Traps
Unidirectional window traps were fixed to window frames for collecting exiting mosquitoes. Each trap consisted of a 30-cm–sided metal frame covered with polyester netting, with 1 side drawn into a funnel to direct mosquitoes into the trap (23). The trap was fixed to a plywood sheet that could be fitted to window frames of differing sizes. The traps were placed before dusk and emptied of mosquitoes at 7
Treatment of Mosquito Nets
Nets were treated with a microencapsulated formulation of lambdacyhalothrin (Icon 10 CS, Syngenta, Basel, Switzerland). The standard rate of 18 mg/m2 was used.
Mosquito Collection
We conducted the trials during May and June 2008 at the southern sites and during July and August 2008 at the northern site. Rooms of selected houses containing untreated nets were fitted with traps and monitored for 5 consecutive nights to assess baseline mosquito density and blood-feeding and death rates. Nets were then treated with lambdacyhalothrin and monitored for 5 additional nights. Houses that attracted too few mosquitoes during baseline monitoring were dropped. Each morning, mosquitoes were collected from the window traps by mouth aspirator and transferred to paper cups and provided with sugar solution. Indoor resting mosquitoes were then collected from white floor sheets after the windows were sealed off and the rooms were sprayed with a nonresidual pyrethroid. Mosquitoes were identified to species and recorded as blood fed or unfed by microscopy. Scoring of blood-feeding rates was pooled for window trap and room collections. Death rates of the exit trap collections were determined after a 24-hour holding period. An. gambiae mosquitoes were identified to species and molecular form by using the method of Favia et al. (24) and genotyped for kdr by using the method of Martinez-Torres et al. (25).
Data Analysis
We assessed the effect of pyrethroid-treated nets on the proportions of An. gambiae blood-feeding or killed mosquitoes using a random effects generalized linear mixed model, recording the proportions of female mosquitoes before treatment as the baseline (control) group and the proportions after treatment as the test group. The model comprised 4 independent variables: treatment, number of holes per net, total area of all holes in the net under test, and number of persons in the household. Random effects in the model also accounted for repeated sampling over several days and the number of persons sleeping in the room. Regional differences in the condition of nets and in household size between the sites with resistant and susceptible mosquitoes were analyzed by using the Wilcoxon rank sum test. All statistical analyses were conducted by using STATA 9 software (STATA Corp., College Station, TX, USA).
Baseline Characteristics of Mosquito Nets and Sleepers
Eleven households at the southern sites (where mosquitoes are resistant) and 5 households at the northern site (where mosquitoes are susceptible) participated in the study. Each household contributed 1 sleeping room and 1 net to the study. Numbers of holes per net recorded at the southern and northern sites did not differ (p = 0.41) (Table 1). The area of holes per net was significantly smaller for nets from the south (p = 0.0013) (Table 2). Household size in the south was twice that in the north (p = 0.025).
Efficacy of Mosquito Nets Before and After Treatments
During the 2-month trial, 692 An. gambiae mosquitoes; 2,271 Culex quinquefasciatus mosquitoes; and small numbers of Mansonia uniformis, An. pharoensis, and Aedes aegypti mosquitoes were collected at the southern sites. At the northern site, 1,856 An. gambiae mosquitoes, 1,051 Mansonia spp. mosquitoes, and small numbers of An. funestus and Ae. aegypti mosquitoes were collected. Only the malaria vector An. gambiae was analyzed further.
The blood-feeding rate of An. gambiae mosquitoes under untreated nets was higher in the north (46%) than in the south (20%) (Table 2), probably because of the larger size of holes in nets in the north. At the northern site (susceptible mosquitoes), the odds of blood feeding were lower after treatment than before treatment with or without adjustment for other covariates (adjusted odds ratio 0.34; 95% CI 0.26–0.44; p<0.001) (Table 2). The overall protective effect of treatment was 66% (95% CI 56%–74%). The OR for nets with smaller and larger areas of holes indicated that ITNs provided similar levels of protection against the susceptible mosquito population regardless of the condition of the nets (Table 2).
At the southern sites, where mosquitoes are resistant, we found no evidence that sleeping under a treated net was more protective than sleeping under an untreated net (adjusted odds ratio 1.14; 95% CI 0.73–1.76; p = 0.566) (Table 2). There was no difference in blood feeding rates between nets that had a higher number and nets that had a lower number of holes. Nor was there any difference between nets that had a higher surface area or lower surface area of holes. These findings indicated that regardless of physical condition, treated nets provided no additional protection over that of untreated nets.
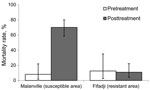
Mosquito mortality rates in the exit traps at the northern site (susceptible mosquitoes) were 8% before insecticide treatment of the nets and 70% after treatment. Mosquito mortality rates at the sites where they are pyrethroid resistant were similar before and after treatment of the nets and did not exceed 12% (Figure).
Species, Molecular, and kdr Genotyping
PCR identified An. gambiae s.s. as the main sibling species at southern and northern sites (Table 3). An. arabiensis mosquitoes were present as a minor sibling species at the northern site. The M form of An. gambiae mosquitoes predominated at all sites (83%). The frequency of kdr was 0.80 in the south and 0.10 in the north.
In this comparative study in areas of contrasting pyrethroid resistance and susceptibility, we used vector blood feeding as a surrogate for malaria risk and demonstrated that ITNs lose their capacity to provide protection once An. gambiae M form develops pyrethroid resistance of the type found in southern Benin (16). These findings clearly show that ITNs in local use fail to protect against An. gambiae populations that contain kdr resistance at high frequency. The mechanisms of resistance in southern Benin are complex, and metabolic resistance appears to contribute (14–16). The demonstration of contrasting blood-feeding and survival rates between resistant and susceptible An. gambiae mosquitoes in the household trial corroborates findings and predictions from earlier experimental hut studies in southern and northern Benin and confirms the veracity of experimental huts as a tool for predicting protection or control in the home (16,22).
We chose to use ordinary household nets rather than new or intact nets. Intact nets might have provided barrier protection against resistant and susceptible mosquitoes, but such a trial would not have reflected local reality. Household nets are inevitably subject to wear and tear, and several studies have documented the association between naturally damaged ITNs and mosquito blood-feeding rates. Before the advent of ITNs, Port and Boreham (26), in an experimental hut study of bed nets previously used by local Gambians, found a strong correlation between blood feeding and the number and size of holes. More recently, Irish et al. (27), in an experimental hut trial of treated nets against pyrethroid-resistant Cx. quinquefasciatus mosquitoes, found an association between the proportion of mosquitoes blood feeding and the number of holes in the ITN. Cross-sectional parasite prevalence surveys in Equatorial Guinea showed that children sleeping under intact ITNs were protected against infection with Plasmodium falciparum but that the level of protection progressively decreased as the nets’ condition deteriorated (28). Our study also stratified nets according to condition, and the analysis showed that persons sleeping under ITNs with holes in areas with pyrethroid-resistant mosquitoes had the same risk from mosquitoes as did persons using untreated nets, whereas in areas of pyrethroid susceptibility, the ITNs remained protective regardless of physical condition. As nets inevitably acquire holes over time, the loss of the nets’ integrity will be felt most strongly in areas with resistant mosquitoes, and the community will be put at greater risk for malaria.
Campaigns of universal LLIN coverage aim to protect the families least able to afford nets (29). With the loss of net integrity over time, malaria transmission will continue across all age groups. Our results predict that mass distribution campaigns of LLINs would benefit populations in areas of pyrethroid susceptibility but are unlikely to control malaria in areas of high resistance. In villages of rural Benin, where pyrethroid resistance in An. gambiae mosquitoes is moderate (kdr frequency averaging 40%), the regular use of LLINs has had some effect on the prevalence of malaria among children <5 years of age (30). We anticipate that in villages with kdr frequency >80% that are subject to high rates of malaria transmission, as in the southern provinces (22,31), the effects on the community of LLINs on malaria would be compromised among families who have poor-quality ITNs.
Sustained protection by any LLIN depends on 2 factors: the rate of loss of insecticide residue from the fibers and the retention of textile integrity. Our research shows that the emphasis placed by the World Health Organization and net manufacturers on developing nets that retain insecticide after recurrent washing is overlooking the role of net durability on effectiveness. A net that retains insecticide after multiple washes or 3 years of use is of no benefit if, before this period, the physical condition of the net and the holes that accumulate mean that in locations with high levels of resistance the net has lost the capacity to protect. During household use, polyester- and polyethylene-based LLINs acquiring holes within the first year and are starting to be discarded after 2 years (21,28,32). LLIN manufacturers need to create new types of fiber or increase the tensile strength to give better resilience against tearing or acquiring holes. Any such product should have a strong commercial advantage.
Resistance capable of undermining the effective use of LLINs is not confined to southern Benin. With the growing coverage of LLINs, the continuing selection of resistance in mosquitoes and its spread to mosquitoes in neighboring provinces and countries is inevitable. Restoring protection of LLINs requires innovation that combines pyrethroids and novel insecticides to which this form of An. gambiae mosquitoes shows no resistance.
Dr Asidi is a postdoctoral scientist based at the Centre de Recherche Entomologique de Cotonou, Benin. His primary research interest is novel types of insecticide for use on ITNs.
Acknowledgments
We thank Renaud Govoetchen and Nazaire Aizoun for technical assistance.
All authors shared in the design of the study, which was co-initiated by C.C. before his sudden death in May 2008 and by M.R. A.A. organized and managed the trial and drafted the manuscript. R.N. supervised the study, analyzed the data and co-wrote the manuscript. M.A. revised the manuscript. M.R .supervised the study, analyzed and interpreted the results, and redrafted the final manuscript. All authors read and approved the final manuscript.
The study was funded by the London School of Hygiene & Tropical Medicine (LSHTM) and through a personal grant from C.C. (Chris Curtis Foundation). LSHTM and the Centre de Recherche Entomologique de Cotonou are members of the Pan African Malaria Vector Research Consortium (www.pamverc.org). A.A., R.N’G., and M.R. are supported by the Malaria Centre of LSHTM.
References
- Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004;CD000363.PubMedGoogle Scholar
- Hill J, Lines J, Rowland M. Insecticide-treated nets. Adv Parasitol. 2006;61:77–128. DOIPubMedGoogle Scholar
- Lines JD, Myamba J, Curtis CF. Experimental hut trials of permethrin-impregnated mosquito nets and eave curtains against malaria vectors in Tanzania. Med Vet Entomol. 1987;1:37–51. DOIPubMedGoogle Scholar
- Magesa SM, Wilkes TJ, Mnzava AE, Njunwa KJ, Myamba J, Kivuyo MD, Trial of pyrethroid impregnated bednets in an area of Tanzania holoendemic for malaria. Part 2. Effects on the malaria vector population. Acta Trop. 1991;49:97–108. DOIPubMedGoogle Scholar
- Chandre F, Manguin S, Brengues C, Dossou Yovo J, Darriet F, Diabate A, Current distribution of a pyrethroid resistance gene (kdr) in Anopheles gambiae complex from west Africa and further evidence for reproductive isolation of the Mopti form. Parassitologia. 1999;41:319–22.PubMedGoogle Scholar
- Sharp BL, Ridl FC, Govender D, Kuklinski J, Kleinschmidt I. Malaria vector control by indoor residual insecticide spraying on the tropical island of Bioko, Equatorial Guinea. Malar J. 2007;6:52. DOIPubMedGoogle Scholar
- Djogbénou L, Pasteur N, Akogbéto M, Weill M, Chandre F. Insecticide resistance in the Anopheles gambiae complex in Benin: a nationwide survey. Med Vet Entomol. 2011;25:256–67. DOIPubMedGoogle Scholar
- Mathias DK, Ochomo E, Atieli F, Ombok M, Bayoh MN, Olang G, Spatial and temporal variation in the kdr allele L1014S in Anopheles gambiae s.s. and phenotypic variability in susceptibility to insecticides in western Kenya. Malar J. 2011;10:10. DOIPubMedGoogle Scholar
- Ranson H, N’Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27:91–8. DOIPubMedGoogle Scholar
- World Health Organization. World malaria report 2011. Geneva: The Organization; 2011.
- Czeher C, Labbo R, Arzika I, Duchemin JB. Evidence of increasing Leu-Phe knockdown resistance mutation in Anopheles gambiae from Niger following a nationwide long-lasting insecticide-treated nets implementation. Malar J. 2008;7:189. DOIPubMedGoogle Scholar
- Ramphul U, Boase T, Bass C, Okedi LM, Donnelly MJ, Müller P. Insecticide resistance and its association with target-site mutations in natural populations of Anopheles gambiae from eastern Uganda. Trans R Soc Trop Med Hyg. 2009;103:1121–6. DOIPubMedGoogle Scholar
- Protopopoff N, Verhaeghen K, Van Bortel W, Roelants P, Marcotty T, Baza D, A significant increase in kdr in Anopheles gambiae is associated with an intensive vector control intervention in Burundi highlands. Trop Med Int Health. 2008;13:1479–87. DOIPubMedGoogle Scholar
- Corbel V, N’Guessan R, Brengues C, Chandre F, Djogbenou L, Martin T, Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin, west Africa. Acta Trop. 2007;101:207–16. DOIPubMedGoogle Scholar
- Djouaka RF, Bakare AA, Coulibaly ON, Akogbeto MC, Ranson H, Hemingway J, Expression of the cytochrome P450s, CYP6P3 and CYP6M2 are significantly elevated in multiple pyrethroid resistant populations of Anopheles gambiae s.s. from southern Benin and Nigeria. BMC Genomics. 2008;9:538. DOIPubMedGoogle Scholar
- N’Guessan R, Corbel V, Akogbéto M, Rowland M. Reduced efficacy of insecticide-treated nets and indoor residual spraying for malaria control in pyrethroid resistance area, Benin. Emerg Infect Dis. 2007;13:199–206. DOIPubMedGoogle Scholar
- N’Guessan R, Asidi A, Boko P, Odjo A, Akogbeto M, Pigeon O, An experimental hut evaluation of PermaNet 3.0, a deltamethrin-piperonyl butoxide combination net, against pyrethroid-resistant Anopheles gambiae and Culex quinquefasciatus mosquitoes in southern Benin. Trans R Soc Trop Med Hyg. 2010;104:758–65. DOIPubMedGoogle Scholar
- Asidi AN, N’Guessan R, Hutchinson RA, Traoré-Lamizana M, Carnevale P, Curtis CF. Experimental hut comparisons of nets treated with carbamate or pyrethroid insecticides, washed or unwashed, against pyrethroid-resistant mosquitoes. Med Vet Entomol. 2004;18:134–40. DOIPubMedGoogle Scholar
- Asidi AN, N’Guessan R, Koffi AA, Curtis CF, Hougard JM, Chandre F, Experimental hut evaluation of bednets treated with an organophosphate (chlorpyrifos-methyl) or a pyrethroid (lambdacyhalothrin) alone and in combination against insecticide-resistant Anopheles gambiae and Culex quinquefasciatus mosquitoes. Malar J. 2005;4:25. DOIPubMedGoogle Scholar
- Henry MC, Assi SB, Rogier C, Dossou-Yovo J, Chandre F, Guillet P, Protective efficacy of lambda-cyhalothrin treated nets in Anopheles gambiae pyrethroid resistance areas of Côte d’Ivoire. Am J Trop Med Hyg. 2005;73:859–64.PubMedGoogle Scholar
- World Health Organization. Report of the Twelfth WHOPES Working Group Meeting, 8–11 December 2008. Geneva: The Organization; 2009.
- Yadouleton AW, Padonou G, Asidi A, Moiroux N, Biol-Banganna S, Corbel V, Insecticide resistance status in Anopheles gambiae in southern Benin. Malar J. 2010;9:83. DOIPubMedGoogle Scholar
- Bar-Zeev M, Self LS. A note on the use of window traps as a tool for evaluating insecticides. Mosq News. 1966;26:205–7.
- Favia G, della Torre A, Bagayoko M, Lanfrancotti A, Sagnon N, Touré YT, Molecular identification of sympatric chromosomal forms of Anopheles gambiae and further evidence of their reproductive isolation. Insect Mol Biol. 1997;6:377–83. DOIPubMedGoogle Scholar
- Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Berge JB, Devonshire AL, Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol. 1998;7:179–84. DOIPubMedGoogle Scholar
- Port GR, Boreham PFL. The effect of bednets on feedng by Anopheles gambiae Giles (Diptera: Culicidae). Bull Entomol Res. 1982;72:483–8. DOIGoogle Scholar
- Irish S, N’Guessan R, Boko PM, Metonnou C, Odjo A, Akogbeto M, Loss of protection with insecticide-treated nets against pyrethroid-resistant Culex quinquefasciatus mosquitoes once nets become holed: an experimental hut study. Parasit Vectors. 2008;1:17. DOIPubMedGoogle Scholar
- Rehman AM, Coleman M, Schwabe C, Baltazar G, Matias A, Gomes I, How much does malaria vector control quality matter: the epidemiological impact of holed nets and inadequate indoor residual spraying. PLoS ONE. 2011;6:e19205. DOIPubMedGoogle Scholar
- Tsuang A, Lines J, Hanson K. Which family members use the best nets? An analysis of the condition of mosquito nets and their distribution within households in Tanzania. Malar J. 2010;9:211. DOIPubMedGoogle Scholar
- Damien GB, Djènontin A, Rogier C, Corbel V, Bangana SB, Chandre F, Malaria infection and disease in an area with pyrethroid-resistant vectors in southern Benin. Malar J. 2010;9:380. DOIPubMedGoogle Scholar
- Nahum A, Erhart A, Mayé A, Ahounou D, van Overmeir C, Menten J, Malaria incidence and prevalence among children living in a peri-urban area on the coast of Benin, west Africa: a longitudinal study. Am J Trop Med Hyg. 2010;83:465–73. DOIPubMedGoogle Scholar
- Kilian A, Byamukama W, Pigeon O, Atieli F, Duchon S, Phan C. Long-term field performance of a polyester-based long-lasting insecticidal mosquito net in rural Uganda. Malar J. 2008;7:49. DOIPubMedGoogle Scholar
Figure
Tables
Cite This Article1Deceased.
Table of Contents – Volume 18, Number 7—July 2012
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Mark Rowland, Department of Disease Control, London School of Hygiene & Tropical Medicine, Keppel St, London WC1E 7HT, UK
Top