Volume 19, Number 3—March 2013
Dispatch
Swine Influenza in Sri Lanka
Cite This Article
Citation for Media
Abstract
To study influenza viruses in pigs in Sri Lanka, we examined samples from pigs at slaughterhouses. Influenza (H3N2) and A(H1N1)pdm09 viruses were prevalent during 2004–2005 and 2009–2012, respectively. Genetic and epidemiologic analyses of human and swine influenza viruses indicated 2 events of A(H1N1)pdm09 virus spillover from humans to pigs.
Data on swine influenza in southern Asia are limited (1–3). Sri Lanka is an island in this region with a human population of 21 million and a swine population of ≈83,785 (4,5). Pigs are not routinely imported into Sri Lanka. Most (61%) swine farms are located in the western costal belt spanning the Puttlam, Gampaha, Colombo, and Kalutara districts. In 2010, for these 4 districts, pig population densities were 7, 15, 12, and 1 animal per km2, respectively (4,5). In 2001, for these districts, the human population densities were 246, 1,539, 3,330, and 677 persons per km2, respectively (6).
During 2004–2005 and 2009–2012, tracheal and nasal swab and serum samples were collected from pigs at government slaughterhouses in Sri Lanka (Table 1). Culture tubes with MDCK cells were inoculated with the swab samples, and 2 blind passages were made. Also, embryonated eggs were inoculated by the allantoic route with swab samples collected during 2004–2005. Virus isolates were subtyped by hemagglutination inhibition (HAI) testing and neuraminidase inhibition testing with reference antiserum as described (7,8), and results were confirmed by sequencing the hemagglutinin and neuraminidase gene segments.
RNA extraction, cDNA synthesis, PCR, genome sequencing (9), and one-step quantitative real-time reverse transcription (RT-PCR) for rapid genotyping of all 8 gene segments (10) of A(H1N1)pdm09 isolates were performed as described. Methods used for the phylogenetic analysis are described in Technical Appendix 1. GenBank accession numbers assigned to the sequences determined in this study are KC197816–KC197855 and KC190041–KC190078.
Serum samples were tested by HAI as described (8) by using the virus antigens shown in Table 2. The number of human influenza A(H1N1)pdm09 viruses detected by RT-PCR in Sri Lanka during July 2009–March 2012 was obtained from the World Health Organization FluNet and from the Epidemiology Unit, Ministry of Health, Sri Lanka (11,12). Seven A(H1N1)pdm09 viruses isolated from humans during 2009–2011 were obtained from the National Center for Influenza, Medical Research Institute, Sri Lanka, and were genetically sequenced as described above. Genetic sequence data of the hemagglutinin gene of 5 additional human A(H1N1)pdm09 viruses isolated in Sri Lanka were provided by the World Health Organization Influenza Collaborating Centre, Melbourne, Australia.
One influenza A virus, A/swine/Colombo/48/2004(H3N2), was isolated in MDCK cells from a tracheal swab sample collected in 2004–2005. All genes of this virus were closely related to human influenza (H3N2) virus isolate A/Ragama/190/2003 from Sri Lanka and to other subtype H3N2 influenza viruses isolated worldwide at this time (data not shown). During January 2004–March 2005, a total of 185 (61.6%) of 300 serum samples tested were positive for A/swine/Colombo/48/2004(H3N2); HAI titers ranged from 40 to >1,280 (Table 2), indicating that this human-like influenza (H3N2) virus was widespread in the swine population. Serum samples collected from swine during 2009–2012 were also mostly seronegative to this and to more contemporary human influenza (H3N2) viruses.
Of the nasal and tracheal swab samples collected from 2,710 pigs during 2009–2012, a total of 26 (0.5%) viruses were isolated in MDCK cells; all were identified as A(H1N1)pdm09 viruses. All 8 gene segments of these viruses were similar to those of A(H1N1)pdm09 virus; no evidence of reassortment with other swine or human viruses was found. These isolates were collected on 12 sampling occasions from apparently healthy pigs on 7 farms. The 2 peaks of A(H1N1)pdm09 detection in swine followed peaks of human A(H1N1)pdm09 outbreaks that occurred during June 2009–January 2010 and October 2010–February 2011, which represented the first and second waves of the pandemic in Sri Lanka (Figure 1). Overall, virus yield was higher from nasal swab samples than from tracheal swab samples (Table 1).
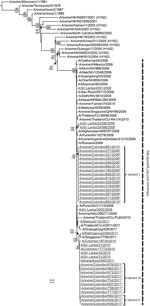
Phylogenetic analysis showed that the 15 A(H1N1)pdm09 viruses isolated from swine during October 2009–July 2010 clustered together and with other A(H1N1)pdm09 viruses isolated from humans during this period. In contrast, swine A(H1N1)pdm09 viruses isolated in 2011 clustered separately from swine viruses isolated during 2009−2010 and clustered with human A(H1N1)pdm09 viruses isolated in 2010 and 2011 in Sri Lanka and elsewhere (Figure 2). The amino acid signature changes occurring within human A(H1N1)pdm09 viruses in the first and second pandemic waves are reflected in the corresponding waves of swine infections, and each linage that occurred in swine led to extinction (Technical Appendix 2). This finding suggests that the A(H1N1)pdm09 infections among swine that occurred during January–February 2011 were separate spillover events from the second wave of human infections during October 2010–February 2011 rather than from continued epizootic transmission among swine from October 2009. The upper 95% CI of the prevalence of each viral genetic variant, given no positive isolates since the last detection, declined to almost zero during the course of observation, indicating probable extinction of these genetic variants (Technical Appendix 2).
On some sampling occasions, A(H1N1)pdm09 viruses were isolated from multiple pigs from the same farm, and on 1 sampling occasion, isolates came from multiple pigs (from the same farm) slaughtered 9 days apart. In such instances, with 1 exception, we found viruses from the same farm to be genetically identical, suggesting continued circulation of the virus in swine herds.
Swine serum samples collected in 2004–2005 showed no seroprevalence to A(H1N1)pdm09, A/California/4/2009, and A/swine/Colombo/330/2009 viruses (Table 2). In 2010, seroprevalence to A(H1N1)pdm09 virus was detected (Figure 1). After peaking in February 2011, seroprevalence declined to undetectable levels in April–May 2012, suggesting that the A(H1N1)pdm09 virus was not sustaining transmission among pigs in the absence of continued human infection. The maximum cross-correlation between incidence of human and swine virus isolates was found after an 8-week lag, indicating that the rise in incidence of human virus preceded that in swine by 7–8 weeks (Technical Appendix 2).
Isolation of human-like influenza A (H3N2) and A(H1N1)pdm09-like viruses from pigs in Sri Lanka probably represents spillover infection from humans, with self-limited transmission and extinction within pig herds. This finding might reflect characteristics of swine husbandry in Sri Lanka, where swine population density in the study area is relatively low (7.7 pigs/km2), or other factors (5,13). Genetic characterization of individual gene segments of all influenza (H3N2) and A(H1N1)pdm09 viruses from swine showed no evidence of genetic reassortment. This finding contrasts with those from Hong Kong, Thailand, Argentina, and the United States, where reassortment of A(H1N1)pdm09 with other swine influenza viruses has reportedly occurred (14,15). This contrast might reflect the low prevalence of other swine influenza virus lineages (e.g., classical swine, Eurasian avian-like and triple-reassortant swine) endemic to Sri Lanka. With the exception of subtype H3N2 viruses (Table 2), no evidence of other endemic swine influenza viruses circulating in swine in the country before the emergence of the A(H1N1)pdm09 in 2009 was found, and influenza (H3N2) virus in swine became extinct around the time of the spillover of A(H1N1)pdm09 to swine. These observations might explain the lack of emergence of A(H1N1)pdm09 reassortants among swine. It might also indicate that A(H1N1)pdm09, although able to spill over from humans to swine, is not ideally adapted to establish sustained transmission among swine in the absence of further reassortment with other swine influenza virus lineages.
Dr Perera is a postgraduate student in the School of Public Health, University of Hong Kong, and a senior lecturer in microbiology at the University of Kelaniya, Sri Lanka. His research focuses on ecology and evolution of influenza viruses.
Acknowledgments
We thank Ian Barr for providing sequence data for human A(H1N1)pdm09 viruses, and we acknowledge the excellent technical assistance of A. G. Premarathne, T. Y. Leung, H. N. Dulani, and M. F. Yeung.
This work was supported in part by National Science Foundation Sri Lanka grant no. RG/2003/M/05 (2004–2005), National Institute of Allergy and Infectious Diseases contract no. HHSN266200700005C, an Area of Excellence Scheme of the University Grants Committee (grant no. AoE/M-12/06) of the Hong Kong Special Administrative Region Government, a PhD studentship from the University of Hong Kong, and by funding support from the Japan Science and Technology Agency Precursory Research for Embryonic Science and Technology project.
References
- Rao BL, Khan FU, Bhat HR, Kadam SS. Zoonotic studies on influenza in pigs and birds, India, 1980–81. Int J Zoonoses. 1983;10:40–4 .PubMedGoogle Scholar
- Nagarajan K, Saikumar G, Arya RS, Gupta A, Somvanshi R, Pattnaik B. Influenza A H1N1 virus in Indian pigs and its genetic relatedness with pandemic human influenza A 2009 H1N1. Indian J Med Res. 2010;132:160–7 .PubMedGoogle Scholar
- Chatterjee S, Mukherjee KK, Mondal MC, Chakravarti SK, Chakraborty MS. A serological survey of influenza A antibody in human and pig sera in Calcutta. Folia Microbiol (Praha). 1995;40:345–8 . DOIPubMedGoogle Scholar
- Abeyrthne AS. A review of the livestock industry of Sri Lanka. Kandy (Sri Lanka): Kandy Printers; 2007. p. 57–65.
- Department of Animal Production and Health Sri Lanka [cited 2012 May 15]. http://www.daph.gov.lk/web/index.php?option=com_content&view=article&id=115&Itemid=104&lang=en
- Department of Census and Statistics Sri Lanka [cited 2012 May 9]. http://www.statistics.gov.lk/PopHouSat/PDF/Population/p9p1%20Growth.pdf
- World Health Organization. WHO manual on animal influenza diagnosis and surveillance [cited 2012 May 15]. http://whqlibdoc.who.int/hq/2002/WHO_CDS_CSR_NCS_2002.5.pdf
- Trevennec K, Leger L, Lyazrhi F, Baudon E, Cheung CY, Roger F, Transmission of pandemic influenza H1N1 (2009) in Vietnamese swine in 2009–2010. Influenza Other Respi Viruses. 2012;6:348–57. DOIPubMedGoogle Scholar
- Huang K, Bahl J, Fan XH, Vijaykrishna D, Cheung CL, Webby RJ, Establishment of an H6N2 influenza virus lineage in domestic ducks in Southern China. J Virol. 2010;84:6978–86 . DOIPubMedGoogle Scholar
- Mak PW, Wong CK, Li OT, Chan KH, Cheung CL, Ma ES, Rapid genotyping of swine influenza viruses. Emerg Infect Dis. 2011;17:691–4. DOIPubMedGoogle Scholar
- World Health Organization. FluNet [cited 2012 May 15]. http://www.who.int/influenza/gisrs_laboratory/flunet/en/
- Epidemiology Unit, Ministry of Health, Sri Lanka, Information on influenza [cited 2012 May 9]. http://www.epid.gov.lk/web/index.php?option=com_content&view=article&id=180&Itemid=494&lang=en
- The Ministry of Public Administration and Home Affairs. Sri Lanka [cited 2012 May 29]. http://www.ds.gov.lk/
- Gray GC, Baker WS. Editorial commentary: the problem with pigs: it’s not about bacon. Clin Infect Dis. 2011;52:19–22. DOIPubMedGoogle Scholar
- Lindstrom S, Garten R, Balish A Shu B, Emery S, Berman L, Human infections with novel reassortant influenza A(H3N2)v viruses, United States, 2011. Emerg Infect Dis. 2012;18:834–7 . DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 19, Number 3—March 2013
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Joseph S.M. Peiris, School of Public Health, 21 Sassoon Rd, University of Hong Kong, Pokfulam, Hong Kong, People’s Republic of China; email:
Top