Volume 21, Number 11—November 2015
CME ACTIVITY - Synopsis
Uncommon Candida Species Fungemia among Cancer Patients, Houston, Texas, USA
Cite This Article
Citation for Media
Introduction

Medscape, LLC is pleased to provide online continuing medical education (CME) for this journal article, allowing clinicians the opportunity to earn CME credit.
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint providership of Medscape, LLC and Emerging Infectious Diseases. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)TM. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/eid; (4) view/print certificate.
Release date: October 15, 2015; Expiration date: October 15, 2016
Learning Objectives
Upon completion of this activity, participants will be able to:
• Distinguish the most prevalent uncommon Candida infection in the current study
• Evaluate trends in bloodstream infections with uncommon Candida infections
• Describe the phenomenon of breakthrough fungemia
• Determine variables associated with a higher risk for mortality among patients with candidemia
CME Editor
Jean Michaels Jones, Technical Writer/Editor, Emerging Infectious Diseases. Disclosure: Jean Michaels Jones has disclosed no relevant financial relationships.
CME Author
Charles P. Vega, MD, Clinical Professor of Family Medicine, University of California, Irvine. Disclosure: Charles P. Vega, MD, has disclosed the following financial relationships: served as an advisor or consultant for Lundbeck, Inc.; McNeil Pharmaceuticals; Takeda Pharmaceuticals North America, Inc.
Authors
Disclosures: Dong Sik Jung, MD; Dimitrios Farmakiotis, MD; Ying Jiang, MS; and Jeffrey J. Tarrand, MD, have disclosed no relevant financial relationships. Dimitrios P. Kontoyiannis, MD, ScD, has disclosed the following relevant financial relationships: served as an advisor or consultant for Merck & Co, Inc., Astellas Pharma US; served as a speaker or a member of a speakers bureau for Merck & Co, Inc., Astellas Pharma US, Gilead Sciences, Inc.; received grants for clinical research from Merck & Co, Inc., Astellas Pharma US, Pfizer.
Abstract
Many uncommon Candida species that cause bloodstream infections (BSIs) are not well-characterized. We investigated the epidemiology, antifungal use, susceptibility patterns, and factors associated with all-cause death among cancer patients in whom uncommon Candida spp. BSIs were diagnosed at a cancer treatment center during January 1998–September 2013. Of 1,395 Candida bloodstream isolates, 79 from 68 patients were uncommon Candida spp. The incidence density of uncommon Candida spp. BSIs and their proportion to all candidemia episodes substantively increased during the study period, and the rise was associated with increasing use of echinocandin antifungal drugs. Thirty-seven patients had breakthrough infections during therapy or prophylaxis with various systemic antifungal drugs for >7 consecutive days; 21 were receiving an echinocandin. C. kefyr (82%), and C. lusitaniae (21%) isolates frequently showed caspofungin MICs above the epidemiologic cutoff values. These findings support the need for institutional surveillance for uncommon Candida spp. among cancer patients.
Despite the widespread use of antifungal prophylaxis and the introduction of new antifungal agents, the incidence of candidemia and associated mortality rates among patients with cancer remain relatively unchanged (1). In previous studies (1–3), >90% of all Candida-associated invasive fungal infections were caused by 1 of 5 Candida spp.: C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, or C. krusei. However, the use of antifungal drugs such as azoles for prophylaxis and echinocandins that are being used more frequently among high-risk populations have been associated with a continuous shift from C. albicans to various non-albicans Candida spp. during the past 2 decades (1,4–9). Moreover, uncommon Candida spp. have emerged as causes of nosocomial bloodstream infections (BSIs) in studies of specific Candida spp. Those isolates commonly exhibit decreased in vitro susceptibility to antifungal agents (10–15).
The epidemiology and clinical features of many uncommon Candida spp. BSIs have not been well characterized. To that end, we evaluated the epidemiologic characteristics, susceptibility patterns, and factors associated with all-cause death among cancer patients who had uncommon Candida spp. BSIs. We also determined whether the increasing frequency of uncommon Candida spp. BSIs in the study cohort correlated with the increased use of specific antifungal agents.
Isolates
In this retrospective study, we examined the clinical microbiology database at the University of Texas MD Anderson Cancer Center (Houston, Texas, USA) to identify blood cultures that were positive for Candida spp. from patients ≥18 years of age during January 1998–September 2013. Candida isolates were grown on Sabouraud dextrose medium (37°C/48 h/200 rpm) and then phenotypically identified by using CHROMagar Candida medium (CHROMagar Company, Paris, France) and VITEK-2 YST (bioMérieux, Marcy l’Etoile, France). The identification methods were not changed during the study period. We excluded unidentified Candida spp. For our analyses, we selected only the first isolate recovered from blood if a patient had several blood cultures drawn that were positive for the same uncommon Candida spp. Antifungal susceptibility was tested by using the Clinical Laboratory Standards Institute broth microdilution reference method (16). The MIC for caspofungin was tested after March 2005 in the center. For uncommon Candida spp., other than for C. guilliermondii, clinical breakpoints are undefined; therefore, isolates that showed MICs higher than the epidemiologic cutoff value (ECV) were considered potentially resistant (17). There was no ECV for C. famata; therefore, those isolates were excluded from susceptibility comparisons.
Data Collection
We retrospectively reviewed the electronic medical records of patients to obtain demographic, clinical, and laboratory data on the day of blood culture collection (Table 1); we also determined 28-day, all-cause mortality rates using a standardized electronic data collection form. Only first episodes of uncommon Candida spp. BSIs per patient were included in survival analyses. The study and a waiver of informed consent for anonymous data collection were approved by the Institutional Review Board of the MD Anderson Cancer Center.
Definitions
An episode of candidemia was defined as signs or symptoms of infection and >1 blood culture that was positive for Candida spp. Episodes were considered to be separate if they occurred ≥1 month apart. Breakthrough candidemia was defined as candidemia in a patient who had undergone therapy or prophylaxis with any systemic antifungal drug for >7 consecutive days before the index blood culture (18).
Neutropenia was defined as an absolute neutrophil count (ANC) of <500/μL, with further stratification at <100. Persistent neutropenia was defined as an ANC of <500 for >7 days. Neutrophil recovery was defined as restoration of the ANC to >500 for >3 consecutive days (18,19). The source of candidemia was considered to be intraabdominal if the patient had undergone abdominal surgery or had gastrointestinal graft-versus-host disease, peritonitis, cholecystitis, or cholangitis.
Catheter-related bloodstream infections were defined as described by Raad et al. (20) as 1) a colony count of blood obtained through the catheter hub that was >5-fold higher than that in blood obtained from a peripheral vein or 2) a catheter tip culture that was positive for Candida spp. The department of pharmacy provided defined daily doses according to the World Health Organization Anatomical Therapeutic Chemical classification system definition (http://www.whocc.no) for echinocandins, azoles, and amphotericin B (ampB) per 1,000 adult inpatient-days during the study period.
Statistical Analysis
We used descriptive statistics to summarize the demographic, clinical, and outcome variables and the in vitro susceptibility data. We compared percentages with the χ2 test or Fisher exact test if the expected numbers were <5 in >20% of all cells. Poisson regression and the Cochran-Armitage test were used for the trend analysis of the annual BSI incidence densities and the proportions of candidemia caused by uncommon Candida spp., respectively. We also compared BSI incidence densities for 2 time periods—1998–2005 and 2006–2013—using Poisson distribution and test-based methods. The correlation between the annual use of antifungals and time was evaluated by using the Spearman correlation. The associations between the incidence densities of uncommon Candida spp. BSIs and the annual use of antifungals (defined as daily doses per 1,000 patient-days) were evaluated by using Poisson regression.
We used Cox regression analysis to identify factors that were significantly associated with death. Clinically relevant parameters in the univariate analyses (p<0.1) were included at model entry. The full model was reduced to a final model by using a stepwise elimination procedure. The proportional hazards assumption was tested graphically and by building time-dependent variables. Two-tailed p values <0.05 were considered statistically significant. All analyses were done by using SPSS statistical software version 21 (SPSS IBM, Armonk, NY, USA).
Incidence Trends and Antifungal Use
We identified 1,395 blood cultures that were positive for Candida over the 16-year study period. We excluded 14 cultures that grew unspecified Candida spp. A total of 79 episodes of illness among 68 patients were caused by 5 uncommon Candida spp.: C. guilliermondii (n = 28, 41%), C. lusitaniae (n = 19, 28%), C. kefyr (n = 13, 19%), C. famata (n = 7, 10%), and C. dublinensis (n = 1, 1%). Patient demographic and clinical characteristics are shown in Table 1. Most patients had hematologic malignancies (n = 51, 75%). Of 44 patients who had low neutrophil counts, 40 were severely neutropenic (91%, ANC <100/μL).
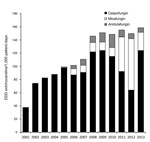
The overall incidence of uncommon Candida spp. BSIs and their proportion relative to all episodes of candidemia increased significantly during 1998–2013 (incidence density p<0.0001; proportion p = 0.001) (Figure 1). The overall incidence density of uncommon Candida spp. BSIs was 3.17 episodes per 100,000 inpatient days, which increased from 1.89 (1998–2005) to 4.2 (2006–2013; p = 0.0001). The overall proportion of uncommon Candida spp. relative to all episodes of candidemia was 5.7% and increased from 3.6% (1998–2005) to 7.2% (2006–2013; p = 0.0004). During 2006–2013, C. lusitaniae had the highest incidence density (1.45 episodes/100,000 inpatient days), followed by C. guilliermondii (1.16), C. kefyr (1.01), and C. famata (0.51). The incidence density of candidemia caused by C. lusitaniae (p = 0.013) and C. kefyr (p = 0.01) increased significantly during 2006–2013 compared with that during 1998–2005; the incidence density of C. guilliermondii BSIs did not increase, and C. famata BSIs showed a trend for increase (p = 0.068) (Figure 1).
Echinocandins became available at the cancer center in 2001. The annual use of echinocandins increased significantly during 2001–2013 (Spearman r = 0.98; p<0.0001) (Figure 2), whereas annual azole and ampB use did not (data not shown). The increase in incidence density of uncommon Candida spp. BSIs was associated with the continuous increase in echinocandin use (p = 0.0062).
Breakthrough Fungemia
Fungemia was detected in samples from 37 of 68 patients (54%) while they were being treated with antifungal agents, specifically with echinocandins (n = 21, 57%), ampB (n = 9, 24%), azoles (n = 6, 16%), or antifungal combinations (n = 1, 3%) (Table 2). Among 6 patients who experienced breakthrough fungemia during treatment with caspofungin, susceptibility data was available for 5 isolates; none were susceptible to caspofungin (MICs 4, 8, 8, 8, and 16 μg/mL). The most common species that caused breakthrough fungemia were C. guilliermondii (16/37 patients, 43%), C. kefyr (8/37 patients, 22%), C. lusitaniae (7/37 patients, 19%), and C. famata (6/37 patients, 16%). Most patients with breakthrough infections had underlying leukemia (33/37, 89%), compared with 9/31 patients (29%) who had no breakthrough infections (p<0.001), and neutropenia (31/37, 84%), compared with 13/31 (42%) who had no breakthrough infections (p<0.001). In addition, more patients who had breakthrough candidemia (26/37, 74%) than de novo candidemia (9/31, 29%) were admitted to the intensive care unit (ICU) (p = 0.001). The crude 28-day mortality rate among patients with breakthrough fungemia was 76% (28/37) (Table 2) and was significantly higher than that for patients with de novo candidemia (12/29, 41%; p = 0.005); Information regarding 28-day survival was available for 29 of 31 patients with de novo candidemia.
In Vitro Susceptibility
In vitro susceptibility results were available for 57 isolates (Table 3). C. guilliermondii strains exhibited high rates of azole MICs above ECVs (fluconazole, 17%; voriconazole and posaconazole, 24%; Table 3). The 2 species that commonly were positive for caspofungin MICs above ECVs were C. kefyr (82% vs. 17% among other species; p<0.001) and C. lusitaniae (21%) (Table 3). Caspofungin MIC clinical breakpoints have been proposed only for C. guilliermondii (17); consequently, 13 C. guilliermondii isolates (87%) were susceptible to caspofungin (MIC <2 μg/mL), 1 was intermediate (MIC = 4 μg/mL), and 1 was resistant (MIC >8 μg/mL). One C. famata isolate had high caspofungin and fluconazole MICs (16 μg/mL for each). Even though ECVs for that species have not been defined, on the basis of ECV and clinical breakpoints for other Candida spp., that isolate could be considered azole/candin-nonsusceptible, making it multidrug resistant.
All-Cause Mortality
The all-cause 28-day mortality rate among this study cohort was 61% (40/66) (Table 4) and was positively associated with underlying leukemia, steroid exposure, ICU stay on the day candidemia was suspected and tested for, intubation, persistent neutropenia, high APACHE II scores (>19), hypoalbuminemia, and breakthrough fungemia (Table 5). We found no statistically significant association between all-cause deaths and specific Candida spp. or central venous catheter removal. In the multivariate Cox regression analysis, an ICU stay (adjusted hazard ratio [aHR] 4, 95% CI 1.8–9.05), persistent neutropenia (aHR 3, 95% CI 1.52–6.05), and a high APACHE II score (>19; aHR 2.8, 95% CI 1.39–5.78) were independently associated with the 28-day all-cause mortality rate (Table 5).
Comprehensive population-based registries of candidemia surveillance have documented the shift from human infections with C. albicans to non-albicans species over the past 2 decades (4,21,22). However, institutional surveillance is equally essential. For example, higher rates of echinocandin resistance are reported from oncology and transplantation centers in the United States (23–25) compared with population-based cohorts (4). At the MD Anderson Cancer Center hospital, the incidence of BSIs caused by uncommon Candida spp. and the proportion of those cases relative to all candidemia cases more than doubled during the past 16 years. Uncommon Candida spp. were frequently nonsusceptible to azoles and echinocandins and were commonly associated with breakthrough infections and high mortality rates. Notably, the incidence density for BSIs caused by uncommon Candida spp. was positively associated with the annual use of echinocandins.
Uncommon Candida spp. distributions vary by geographic region, patient population, and antifungal practices. In general, reported frequencies have been <10% among all Candida isolates (21,22,26,27), which is similar to the proportion of uncommon Candida spp. among all Candida BSIs (3.6%) during the first period of our study (1998–2005) and to that (3.3%) found in another study of cancer patients during 2009–2012 (28). Nevertheless, the proportion of uncommon Candida spp. BSIs relative to all episodes of candidemia in the MD Anderson Cancer Center hospital increased over the years, accounting for 12% of all Candida BSIs reported during 2013 (Figure 1), which is among the highest proportions reported to date. This striking difference reflects a severely immunocompromised patient population: 75% had hematologic malignancies, compared with 10.7% in the study by Tang et al (28). However, the most crucial determinant of this marked increase in uncommon Candida BSIs is likely the broad use of echinocandins. For example, in the study by Tang et al. (28), 88.8% of cancer patients with candidemia had previously received fluconazole and <2% had received an echinocandin; the opposite was true in our cohort, where almost one third of patients with uncommon Candida spp. fungemia had breakthrough infections while being treated with an echinocandin. Moreover, the incidence density of the uncommon Candida spp. BSIs in our study was positively associated with the increase in treatment with echinocandins.
In previous reports, C. guilliermondii was one of the most commonly isolated uncommon Candida spp. among patients with cancer (9,13,29); C. dubliniensis was common in the outpatient setting (27). Nevertheless, in our study, during the years 2006–2013, C. guilliermondii was not the most common isolate, and the incidence of C. guilliermondii fungemia did not increase substantially over the study period (Figure 2). This finding is in agreement with another study, wherein the increased use of echinocandins was not associated with an increase in the incidence of C. guilliermondii fungemia (30). The increase in the incidence of C. kefyr, predominantly among patients with hematologic malignancies, is in agreement with the results of another recent report (12), in which the increase was also attributed to the increasing use of the echinocandin drug micafungin. Taken together, those findings highlight the need, at an institutional level, to systematically monitor changes in Candida spp. distribution and the association with the selective pressure from antifungals.
The clinical features and outcomes of breakthrough candidemia with uncommon Candida spp. have not been well described. In our study, more than half of all patients with fungemia caused by uncommon Candida spp., and 36 of 51 patients who had hematologic malignancies (70%), had breakthrough infections. On the contrary, in a 1993–1998 candidemia study at our institution in which uncommon Candida spp. were excluded, ≈25% of all patients, and 46% of those with hematologic malignancies, had breakthrough infections (31). Nevertheless, the percentage of breakthrough infections among all Candida spp. BSIs (53%) in a more recent report (32) was almost identical to that in this study of fungemia caused by uncommon Candida spp. (54%). Those differences are further reflective of the changing epidemiologic characteristics of candidemia and the unique features of uncommon Candida spp. breakthrough infections, which seem to affect a more compromised patient population.
A direct comparison between common and uncommon Candida spp. was beyond the scope of this study, but in another report, among candidemic patients with acute leukemia, we observed a trend for higher mortality rates with the same uncommon Candida spp. infections on univariate analysis, but not on multivariate analysis (25). The only independent predictors of death in the study described here were ICU stay, persistent neutropenia, and high APACHE II score (Table 5), confirming that host characteristics are the most powerful predictors of response and should be adequately adjusted for in studies of candidemia outcomes.
We used the ECV to characterize uncommon Candida spp. bloodstream isolates as susceptible or potentially resistant, according to the updated Clinical and Laboratory Standards Institute/EUCAST definitions (17). C. guilliermondii strains exhibited high rates of azole resistance (Table 3), in agreement with the results of previous reports (13,33,34). However, echinocandin resistance among C. guilliermondii bloodstream isolates in our study was uncommon (a MIC >1 mg/L was observed for only 13% of isolates); in contrast, Girmenia et al. reported that a caspofungin MIC >1 mg/L was observed for 67% of C. guilliermondii strains (13). Moreover, the incidence of C. guilliermondii BSIs remained stable during the 16 years of our study (Figure 1) and was not substantially associated with echinocandin use. On the contrary, the most common species with caspofungin MICs above ECV was C. kefyr (82%); the incidence density for the species increased substantially over time (Figure 1) and was positively associated with the annual use of echinocandins (p = 0.004), but not azoles or ampB. Dufresne et al. (12) recently reported a similar rate (88%) of micafungin resistance (MIC >0.12 mg/L) in C. kefyr bloodstream isolates in patients with hematologic malignancies, possibly associated with institutional use of micafungin.
Our study has limitations that should be taken into consideration. First, it was a retrospective study from a single cancer center with a small number of episodes caused by individual uncommon Candida spp.; therefore, our observations might not be applicable to different patient groups at risk for uncommon Candida spp. BSIs. Second, uncommon Candida spp. were identified phenotypically, and it is possible that during the study period, some C. dublinensis isolates were identified as C. albicans, underestimating the frequency of that species. It should also be noted that with the introduction of molecular identification, the taxonomy of the Candida genus is in a state of change (35). The recent implementation of internal transcriber section sequencing (http://www.cbs.knaw.nl/databases, http://www.ncbi.nlm.nih.gov/genbank) and matrix-assisted laser desorption/ionization in mass spectrometry for Candida spp. identification are expected to further advance understanding of the epidemiology and clinical course of serious infections with uncommon Candida spp.
Third, we used in vitro caspofungin MIC alone to define echinocandin resistance, using no data on DNA mutations. However, there is evidence that caspofungin MIC interlaboratory variability may lead to incorrect categorization of susceptibility results (36), and micafungin and anidulafungin MICs correlate better with the presence of FKS mutations and clinical outcomes (37). Resistance to echinocandins emerges as a result of treatment and has been associated with mutations in FKS 1/2 genes, which encode the target enzyme for this specific class of antifungals, β-
In summary, we observed a marked increase in the frequency of BSIs caused by uncommon Candida spp. in a contemporary series of patients with malignancies; those species were often associated with breakthrough infections and high mortality rates. The positive correlation between the increasing incidence of uncommon, potentially resistant Candida bloodstream isolates and the increasing use of echinocandins underscores the need for institutional surveillance and the rational use of antifungal drugs in cancer patients.
Dr. Jung was a visiting professor at the Departments of Infectious Diseases, Infection Control and Employee Health, the University of Texas MD Anderson Cancer Center, at the time of this study. He is currently an associate professor and infectious disease physician at the Dong-A University Hospital. His research interests are fungal infections in immunocompromised patients with cancer and transplant recipients.
Acknowledgments
We thank Cai Wu for providing information for annual use of antifungal agents.
D.P.K. dedicates this article to the loving memory of his twin brother, who recently succumbed to cancer.
D.P.K. is the Frances King Black Endowed Professor for Cancer Research and has received research support and honoraria from Pfizer; Astellas. Pharma US; Gilead Sciences, Inc.; and Merck & Co., Inc. All other authors report no potential conflicts.
References
- Sipsas NV, Lewis RE, Tarrand JJ, Hachem R, Rolston KV, Raad II, Candidemia in patients with hematologic malignancies in the era of new antifungal agents (2001–2007): stable incidence but changing epidemiology of a still frequently lethal infection. Cancer. 2009;115:4745–52. DOIPubMedGoogle Scholar
- Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol. 2010;36:1–53. DOIPubMedGoogle Scholar
- Marchetti O, Bille J, Fluckiger U, Eggimann P, Ruef C, Garbino J, Epidemiology of candidemia in Swiss tertiary care hospitals: secular trends, 1991–2000. Clin Infect Dis. 2004;38:311–20 .DOIPubMedGoogle Scholar
- Cleveland AA, Farley MM, Harrison LH, Stein B, Hollick R, Lockhart SR, Changes in incidence and antifungal drug resistance in candidemia: results from population-based laboratory surveillance in Atlanta and Baltimore, 2008–2011. Clin Infect Dis. 2012;55:1352–61. DOIPubMedGoogle Scholar
- Pfaller MA, Andes DR, Diekema DJ, Horn DL, Reboli AC, Rotstein C, Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2,496 patients: data from the prospective antifungal therapy (PATH) Registry 2004–2008. PLoS ONE. 2014;9:e101510. DOIPubMedGoogle Scholar
- Marr KA, Seidel K, White TC, Bowden RA. Candidemia in allogeneic blood and marrow transplant recipients: evolution of risk factors after the adoption of prophylactic fluconazole. J Infect Dis. 2000;181:309–16. DOIPubMedGoogle Scholar
- Blanchard E, Lortholary O, Boukris-Sitbon K, Desnos-Ollivier M, Dromer F, Guillemot D, Prior caspofungin exposure in patients with hematological malignancies is a risk factor for subsequent fungemia due to decreased susceptibility in Candida spp.: a case-control study in Paris, France. Antimicrob Agents Chemother. 2011;55:5358–61. DOIPubMedGoogle Scholar
- van Burik JH, Leisenring W, Myerson D, Robert CH, Howard MS, George ES, The effect of prophylactic fluconazole on the clinical spectrum of fungal diseases in bone marrow transplantation recipients with special attention to hepatic candiasis: an autopsy study of 355 patients. Medicine. 1998;77:246–54. DOIPubMedGoogle Scholar
- Wingard JR. Importance of Candida species other than C. albicans as pathogens in oncology patients. Clin Infect Dis. 1995;20:115–25 . DOIPubMedGoogle Scholar
- Atkinson BJ, Lewis RE, Kontoyiannis DP. Candida lusitaniae fungemia in cancer patients: risk factors for amphotericin B failure and outcome. Med Mycol. 2008;46:541–6. DOIPubMedGoogle Scholar
- Reuter CW, Morgan MA, Bange FC, Gunzer F, Eder M, Hertenstein B, Candida kefyr as an emerging pathogen causing nosocomial bloodstream infections in neutropenic leukemia patients. Clin Infect Dis. 2005;41:1365–6 . DOIPubMedGoogle Scholar
- Dufresne SF, Marr KA, Sydnor E, Staab JF, Karp JE, Lu K, Epidemiology of Candida kefyr in patients with hematologic malignancies. J Clin Microbiol. 2014;52:1830–7. DOIPubMedGoogle Scholar
- Girmenia C, Pizzarelli G, Cristini F, Barchiesi F, Spreghini E, Scalise G, Candida guilliermondii fungemia in patients with hematologic malignancies. J Clin Microbiol. 2006;44:2458–64 . DOIPubMedGoogle Scholar
- Mardani M, Hanna HA, Girgawy E, Raad I. Nosocomial Candida guilliermondii fungemia in cancer patients. Infect Control Hosp Epidemiol. 2000;21:336–7 . DOIPubMedGoogle Scholar
- Johnson EM. Rare and emerging Candida species. Current Fungal Infection Reports. 2009;3:152–9.
- Clinical Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts, 4th ed. M27–S4. Wayne (PA): The Institute. 2012.
- Pfaller MA, Diekema DJ. Progress in antifungal susceptibility testing of Candida spp. by use of Clinical and Laboratory Standards Institute broth microdilution methods, 2010 to 2012. J Clin Microbiol. 2012;50:2846–56 PubMed. DOIPubMedGoogle Scholar
- Antoniadou A, Torres HA, Lewis RE. Candidemia in a tertiary care cancer center: in vitro susceptibility and its association with outcome of initial antifungal therapy. Medicine. 2003;82:309–21. DOIPubMedGoogle Scholar
- Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of America. Clin Infect Dis. 2011;52:e56–93. DOIPubMedGoogle Scholar
- Raad I, Hanna H, Boktour M, Girgawy E. Management of central venous catheters in patients with cancer and candidemia. Clin Infect Dis. 2004;38:1119–27. DOIPubMedGoogle Scholar
- Falagas ME, Roussos N, Vardakas KZ. Relative frequency of albicans and the various non-albicans Candida spp. among candidemia isolates from inpatients in various parts of the world: a systematic review. Int J Infect Dis. 2010;14:e954–66. DOIPubMedGoogle Scholar
- Pfaller M, Neofytos D, Diekema D, Azie N, Meier-Kriesche HU, Quan SP, Epidemiology and outcomes of candidemia in 3648 patients: data from the Prospective Antifungal Therapy (PATH Alliance®) registry, 2004–2008. Diagn Microbiol Infect Dis. 2012;74:323–31 and. DOIPubMedGoogle Scholar
- Farmakiotis D, Tarrand JJ, Kontoyiannis DP. Drug-resistant Candida glabrata infection in cancer patients. Emerg Infect Dis. 2014;20:1833–40. DOIPubMedGoogle Scholar
- Arendrup MC, Perlin DS. Echinocandin resistance: an emerging clinical problem? Curr Opin Infect Dis. 2014;27:484–92. DOIPubMedGoogle Scholar
- Wang E, Farmakiotis D, Yang D, McCue DA, Kantarjian HM, Kontoyiannis DP, The ever-evolving landscape of candidaemia in patients with acute leukaemia: non-susceptibility to caspofungin and multidrug resistance are associated with increased mortality. J Antimicrob Chemother. 2015;70:2362–8. DOIPubMedGoogle Scholar
- Chen SC, Marriott D, Playford EG, Nguyen Q, Ellis D, Meyer W, Candidemia with uncommon Candida species: predisposing factors, outcome, antifungal susceptibility, and implications for management. Clin Microbiol Infect. 2009;15:662–9 . DOIPubMedGoogle Scholar
- Jung SI, Shin JH, Song JH, Peck KR, Lee K, Kim MN, Multicenter surveillance of species distribution and antifungal susceptibilities of Candida bloodstream isolates in South Korea. Med Mycol. 2010;48:669–74 PubMed. DOIPubMedGoogle Scholar
- Tang HJ, Liu WL, Lin HL, Lai CC. Epidemiology and prognostic factors of candidemia in cancer patients. PLoS ONE. 2014;9:e99103 . DOIPubMedGoogle Scholar
- Kovacicová G, Spanik S, Kunova A, Trupl J, Sabo A, Koren P, Prospective study of fungaemia in a single cancer institution over a 10-y period: aetiology, risk factors, consumption of antifungals and outcome in 140 patients. Scand J Infect Dis. 2001;33:367–74. DOIPubMedGoogle Scholar
- Lai CC, Chu CC, Wang CY, Tsai HY, Cheng A, Lee YC, Association between incidence of candidaemia and consumption of antifungal agents at a medical centre in Taiwan. Int J Antimicrob Agents. 2012;40:349–53 PubMed. DOIPubMedGoogle Scholar
- Kontoyiannis DP, Reddy BT, Hanna H, Bodey GP, Tarrand J, Raad I. Breakthrough candidemia in patients with cancer differs from de novo candidemia in host factors and Candida species but not intensity. Infect Control Hosp Epidemiol. 2002;23:542–5. DOIPubMedGoogle Scholar
- Gamaletsou MN, Walsh TJ, Zaoutis T, Pagoni M, Kotsopoulou M, Voulgarelis M, A prospective, cohort, multicentre study of candidaemia in hospitalized adult patients with haematological malignancies. Clin Microbiol Infect. 2014;20:O50–7 . DOIPubMedGoogle Scholar
- Pfaller MA, Diekema DJ, Mendez M, Kibbler C, Erzsebet P, Chang SC, Candida guilliermondii, an opportunistic fungal pathogen with decreased susceptibility to fluconazole: geographic and temporal trends from the ARTEMIS DISK antifungal surveillance program. J Clin Microbiol. 2006;44:3551–6 . DOIPubMedGoogle Scholar
- Savini V, Catavitello C, Onofrillo D, Masciarelli G, Astolfi D, Balbinot A, What do we know about Candida guilliermondii? A voyage throughout past and current literature about this emerging yeast. Mycoses. 2011;54:434–41 PubMed. DOIPubMedGoogle Scholar
- Fekkar A, Dannaoui E, Meyer I, Imbert S, Brossas JY, Uzunov M, Emergence of echinocandin-resistant Candida spp. in a hospital setting: a consequence of 10 years of increasing use of antifungal therapy? Eur J Clin Microbiol Infect Dis. 2014;33:1489–96. DOIPubMedGoogle Scholar
- Espinel-Ingroff A, Arendrup MC, Pfaller MA, Bonfietti LX, Bustamante B, Canton E, Interlaboratory variability of caspofungin MICs for Candida spp. using CLSI and EUCAST methods: should the clinical laboratory be testing this agent? Antimicrob Agents Chemother. 2013;57:5836–42. DOIPubMedGoogle Scholar
- Shields RK, Nguyen MH, Press EG, Updike CL, Clancy CJ. Anidulafungin and micafungin MIC breakpoints are superior to that of caspofungin for identifying FKS mutant Candida glabrata strains and echinocandin resistance. Antimicrob Agents Chemother. 2013;57:6361–5. DOIPubMedGoogle Scholar
- Kanafani ZA, Perfect JR. Antimicrobial resistance: resistance to antifungal agents: mechanisms and clinical impact. Clin Infect Dis. 2008;46:120–8 . DOIPubMedGoogle Scholar
- Staab JF, Neofytos D, Rhee P, Jimenez-Ortigosa C, Zhang SX, Perlin DS, Target enzyme mutations confer differential echinocandin susceptibilities in Candida kefyr. Antimicrob Agents Chemother. 2014;58:5421–7 . DOIPubMedGoogle Scholar
Figures
Tables
Follow Up
Earning CME Credit
To obtain credit, you should first read the journal article. After reading the article, you should be able to answer the following, related, multiple-choice questions. To complete the questions (with a minimum 75% passing score) and earn continuing medical education (CME) credit, please go to http://www.medscape.org/journal/eid. Credit cannot be obtained for tests completed on paper, although you may use the worksheet below to keep a record of your answers. You must be a registered user on Medscape.org. If you are not registered on Medscape.org, please click on the “Register” link on the right hand side of the website to register. Only one answer is correct for each question. Once you successfully answer all post-test questions you will be able to view and/or print your certificate. For questions regarding the content of this activity, contact the accredited provider, CME@medscape.net. For technical assistance, contact CME@webmd.net. American Medical Association’s Physician’s Recognition Award (AMA PRA) credits are accepted in the US as evidence of participation in CME activities. For further information on this award, please refer to http://www.ama-assn.org/ama/pub/about-ama/awards/ama-physicians-recognition-award.page. The AMA has determined that physicians not licensed in the US who participate in this CME activity are eligible for AMA PRA Category 1 Credits™. Through agreements that the AMA has made with agencies in some countries, AMA PRA credit may be acceptable as evidence of participation in CME activities. If you are not licensed in the US, please complete the questions online, print the certificate and present it to your national medical association for review.
Article Title: Uncommon Candida Species Fungemia among Cancer Patients, Houston, Texas, USA
CME Questions
1. You are seeing a neutropenic 65-year-old woman admitted to the hospital for fever while receiving chemotherapy for acute lymphocytic leukemia. Her initial blood culture results demonstrate growth of a Candida species. Which of the following Candida species was most prevalent among uncommon Candida species causing bloodstream infections in the current study?
A. C. famata
B. C. dubliniensis
C. C. lusitaniae
D. C. guilliermondii
2. Which of the following trends was noted among cases of uncommon Candida bloodstream infections in the current study?
A. A minority of patients had hematologic malignant diseases
B. The proportion of cases of candidemia resulting from uncommon Candida species increased from 1998 to 2013
C.. The incidence of infection with C. guilliermondii increased more than any other species from 2006 to 2013
D. There was no relationship between infections with uncommon Candida species and the use of echinocandins
3. The patient had been treated with an echinocandin as prophylaxis. Which of the following statements regarding breakthrough fungemia in the current study is most accurate?
A. Fungemia was very rare among patients treated with antifungal prophylaxis
B. Most cases of breakthrough fungemia were among patients taking an azole
C. The most common species causing breakthrough fungemia was C. lusitaniae
D. Breakthrough fungemia was associated with higher rates of admission to the intensive care unit and higher mortality rates
4. The patient experiences severe complications associated with this infection. Which of the following variables is most significant as a risk factor for 28-day mortality in the current study?
A. Persistent neutropenia
B. Age older than 60 years
C. Infection with C. guilliermondii in particular
D. Failure to remove a central venous catheter
Activity Evaluation
|
1. The activity supported the learning objectives. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
2. The material was organized clearly for learning to occur. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
3. The content learned from this activity will impact my practice. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
|
4. The activity was presented objectively and free of commercial bias. |
||||
|
Strongly Disagree |
|
|
|
Strongly Agree |
|
1 |
2 |
3 |
4 |
5 |
1These authors contributed equally to this article.
2Current affiliation: Warren Alpert Medical School of Brown University, Providence, Rhode Island, USA.
Related Links
Table of Contents – Volume 21, Number 11—November 2015
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Dimitrios P. Kontoyiannis, T. Boone Pickens Academic Tower, FCT12.5070, 1515 Holcombe Blvd, Houston, TX 77030-4095, USA
Top