Volume 21, Number 3—March 2015
Research
Multidrug-Resistant Tuberculosis in Europe, 2010–2011
Cite This Article
Citation for Media
Abstract
Drug-resistant Mycobacterium tuberculosis is challenging elimination of tuberculosis (TB). We evaluated risk factors for TB and levels of second-line drug resistance in M. tuberculosis in patients in Europe with multidrug-resistant (MDR) TB. A total of 380 patients with MDR TB and 376 patients with non–MDR TB were enrolled at 23 centers in 16 countries in Europe during 2010–2011. A total of 52.4% of MDR TB patients had never been treated for TB, which suggests primary transmission of MDR M. tuberculosis. At initiation of treatment for MDR TB, 59.7% of M. tuberculosis strains tested were resistant to pyrazinamide, 51.1% were resistant to ≥1 second-line drug, 26.6% were resistant to second-line injectable drugs, 17.6% were resistant to fluoroquinolones, and 6.8% were extensively drug resistant. Previous treatment for TB was the strongest risk factor for MDR TB. High levels of primary transmission and advanced resistance to second-line drugs characterize MDR TB cases in Europe.
Emergence of drug-resistant tuberculosis (TB) threatens the goal of TB elimination (1). Multidrug-resistant (MDR) TB is defined by in vitro resistance of Mycobacterium tuberculosis to at least both of the 2 most effective drugs for treatment (rifampin and isoniazid). Extensively drug-resistant TB (XDR TB) is defined as MDR TB plus in vitro resistance to at least 1 second-line injectable drug (amikacin, capreomycin, or kanamycin) plus resistance to any of the fluoroquinolones (e.g., ofloxacin, levofloxacin, or moxifloxacin) (2). In the World Health Organization (WHO) European Region, the estimated incidence of patients with MDR TB differs markedly: 1.6 cases/100,000 persons in the 29 European Union/European Economic Area countries and 16.8 cases/100,000 persons in the 24 other countries of the region in 2012 (Technical Appendix Table 1) (3). The actual number of patients with MDR TB living in this region may be much higher because a substantial proportion of patients are never screened for drug-resistant TB before starting treatment, partly because of a lack of diagnostic capacity (3).
MDR TB is associated with poor treatment outcomes (1,2,4). The proportion of treatment success in patients with MDR TB was only 54% in an individual patient data metaanalysis of >9,000 patients from 32 observational studies (5). Results from this cohort showed that additional resistance to fluoroquinolones in patients with MDR TB reduced treatment success to 48%; patients with XDR TB were treated successfully in 40% of cases (6), which approached treatment outcomes similar to those of the pre–antimicrobial drug era (4). A recent surveillance report from the EU reported 32.2% treatment success for MDR TB and 19.1% treatment success for XDR TB (7).
Detailed information about characteristics, management, and outcomes of patients with MDR TB in Europe is scarce but essential to inform health policy makers and optimize disease management (8). We compared baseline characteristics and risk factors for patients with MDR TB, as well as availability and results of drug susceptibility testing (DST) for second-line drugs for treatment of TB, in a cohort of patients from 16 countries in Europe with low, intermediate, and high incidence of TB, who had started first-line or second-line TB treatment.
Participating Sites
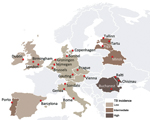
TBNET is a European consortium for clinical research in the field of TB (9). This study was conducted at 23 TBNET sites in 16 countries in Europe: 2 with a high (>100 cases/100,000 persons) incidence of TB, 4 with an intermediate (20–100 cases/100,000 persons) incidence, and 10 with a low (<20 cases/100,000 persons) incidence (Figure). Stratification was based on WHO/European Centre for Disease Prevention and Control estimates of TB incidence during the study period (2010–2011) (10). The number of study participants per country is shown in Technical Appendix Table 1.
Study Population
After informed consent was obtained, patients starting treatment for a new episode of culture-confirmed TB with resistance to at least rifampin and isoniazid (MDR TB cohort) were eligible for enrollment. Patients were included prospectively by using consecutive inclusion during January 2010–December 2011 at each site. In Belarus, Latvia, Moldova, and Romania, additional enrollment was stopped when a preagreed number of patients were enrolled to avoid overrepresentation of patients from these countries in the cohort.
For each MDR TB patient, 1 patient with non–MDR TB (pan drug–susceptible, monoresistant, or polydrug-resistant TB [11]) was enrolled at each center at the time of enrollment of the MDR TB patient: these additional patients were denoted as controls. Controls were selected on the basis of DST results that identified non–MDR TB, and that were obtained at the closest date before enrollment of an MDR TB patient at the same site.
Because of this selection process, a limited number of patients (41, 5.4%) started treatment before the study began in January 2010, but none started treatment before January 2007. However, we maintained consecutive inclusion for MDR TB patients. This feature ensured an acceptable sample size for countries with a low incidence of TB during the inclusion period.
Data Collection
Data collection used an electronic case record form designed in Open Clinica (http://www.openclinica.com). A paper version of this form was used in Moldova, Romania, Estonia, and Belarus, where internet access was not always available. All investigators were initially trained onsite, and continuous training was ensured through annual investigator meetings, regular site visits, and newsletters.
Laboratory Testing
Routine data were obtained from local laboratory reports for sputum smear microscopy, sputum culture, and DST for first-line and second-line drugs and, when available, M. tuberculosis–specific nucleic acid amplification tests. All laboratories at study sites were subjected to quality control through the WHO Supranational Reference Laboratory Network.
Study Outcome
We analyzed characteristics of the cohort at the time of enrollment. We also assessed factors associated with MDR TB in a cross-sectional approach.
Data Management
Data management included regular data checks on key variables for missing data and inconsistencies. The study coordinator, a study monitor, and a trained study nurse performed routinely manual plausibility checks and clarified inconsistencies with the investigators.
Statistical Analysis
Descriptive statistics are reported as frequencies or medians, where appropriate. Risk factor analysis was performed by using univariable and multivariable logistic regression. We used robust SEs to adjust for clustering by country. All variables with <20% missing data were assessed for inclusion in the models. Missing data for included variables were coded as separate categories to prevent listwise deletion of observations. Age was dichotomized at 45 years to align with values in a previous study (12). The variables age and sex were purposefully included in the multivariable analysis in which other variables were included on the basis of the Wald statistic (<0.1) and the change in the pseudo R2 (>10%) because a robust SE precludes formal use of the log-likelihood ratio test. In a sensitivity analysis, we repeated multivariable logistic regression with the inclusion of a sampling weight for the MDR TB patients (inverse of the sampling fraction with expected number of MDR TB patients in the country as denominator) (Technical Appendix Table 5). Non–MDR TB patients were given a weight of 1. The weighted analyses assessed the potential effect of unbalanced contribution of countries in the cohort. Goodness-of-fit was assessed by using the F-adjusted mean residual test.
Drug resistance was expressed as the proportion of isolates tested and the proportion of isolates that were resistant. Corresponding frequencies when applying sampling weights and analysis by a complex survey approach (13,14) are shown in Technical Appendix Table 4.
Ethics
Patient information and consent forms were translated into local languages when needed. The study was approved by the Ethics Committee of the University of Lübeck (Lübeck, Germany). The study protocol was approved by the local ethics committee at all participating centers. Written informed consent was obtained from all patients according to site-specific regulations. Data were collected pseudonymously and stored on a secured server.
Cohort Characteristics
The cohort consisted of 380 MDR TB patients and 376 non–MDR TB controls. Descriptive characteristics of the MDR TB cohort are shown in Table 1 and those for the non–MDR TB cohort in Technical Appendix Table 2. Both groups had predominantly male patients. The median age was 36 years (interquartile range 27–47 years) for the MDR TB patients and 41 years (interquartile range 31–54 years) for the controls. The proportion of foreign-born patients with MDR TB in countries of low, intermediate, and high TB incidence was 85.4%, 5.8% and 0.5%, respectively. Similar proportions were observed in controls (56.3%, 5.7% and 2.1%, respectively). Of 94 foreign-born patients, 60 (64%) were from countries of the European region of WHO, 17 (18%) from Russia, 18 (19%) from Southeast Asia, 11 (12%) from sub-Saharan Africa, 1 (1%) from North Africa, and 4 (4%) from South America. Smoking was common in both groups (50.5% for MDR TB patients and 41.5% for controls).
HIV infection and diabetes mellitus were infrequently observed: 6.6% in MDR TB patients and 4.3% in controls for HIV, and 4.2% in MDR TB patients and 5.3% in controls for diabetes mellitus. The percentage of patients with MDR TB whose episode of active TB was their first was 52.4% (59.2%, 74.4%, and 38.7% in countries with low, intermediate, and high TB incidence, respectively).
Drug Resistance Profiles
Among 380 patients with MDR TB, second-line M. tuberculosis DST profiles were available for 356 patients. Reasons for unavailable baseline DST results were 1) an initial diagnosis of MDR TB at a peripheral hospital and subsequent patient transfer to a central hospital where M. tuberculosis could not be grown in culture (n = 6); 2) contamination of cultures (n = 12); 3) insufficient growth in cultures (n = 4); 4) patient death between the first and second cultures (n = 1), and 5) unknown reason (n = 1). Among patients with MDR TB, 6.8% of cases fulfilled the definition of XDR TB. Drug resistance profiles for first-line and second-line drugs other than rifampin and isoniazid are shown in Table 2 for the MDR TB cohort, in Technical Appendix Table 3 for the MDR TB cohort compared with the non–MDR TB cohort, and in Technical Appendix Table 4 for the MDR TB cohort by weighted analysis.
DST for pyrazinamide and ethambutol was performed for 45.0% (177/380) and 97.6% (371/380) of strains from MDR TB patients and controls, respectively. Testing was performed for 94.7% (360/380) of strains for resistance to any second-line drug, 93.7% (356/380) for any second-line injectable drug, 92.6% (352/380) for any fluoroquinolone, and 93.2% (356/380) for ethionamide/prothionamide. Strains from MDR TB patients showed additional resistance to pyrazinamide (59.7%, 105/177), ethambutol (59.3%, 220/371), ≥1 second-line injectable drug (26.1%, 93/356), ≥1 fluoroquinolone (17.6%, 62/352), and ethionamide/prothionamide (31.3%, 119/354) (Table 2). The weighted analysis showed higher proportions of resistance to all drugs, except capreomycin, moxifloxacin, and ethionamide/prothionamide (Technical Appendix Table 4).
Risk Factors for MDR TB
Risk factors for TB were compared between patients with MDR TB and controls. Previous treatment for TB (odds ratio 10.7, 95% CI 7.3–15.6) and age <45 years (OR 1.90, 95% CI 1.23–2.93) were identified as independent risk factors for MDR TB by multivariable analysis (Table 3). There was also a moderate association for sex and current homelessness with MDR TB by weighted analysis (Technical Appendix Table 5). No association was found between MDR TB and abnormal body mass index (<18 or >25), employment status, birth in a foreign country, history of imprisonment, injectable drug use, co-infection with HIV, or diabetes. The role of TB contact was not evaluated because data were not sufficiently robust because of a high percentage of unknown/unreliable results for self-reporting. Weighted analyses showed similar results with only minor differences in effect size.
We studied a multicenter cohort of patients with MDR TB at 23 referral centers across Europe and found high rates of drug resistance to second-line drugs for treatment of TB in circulating M. tuberculosis strains, and limited availability of second-line drug resistance testing in several countries with a high incidence of TB. Furthermore, we found evidence of ongoing transmission of MDR strains of M. tuberculosis in eastern Europe: 52.4% of patients with MDR TB were experiencing their first episode of TB. In countries in western Europe with a low incidence of TB, MDR TB is predominantly a disease of immigrants (15), which reflects the epidemiology of MDR TB in the country of origin. Only a few (8.9%) MDR TB patients were born outside the European region of WHO. Thus, interventions for the control of MDR TB should be specific for countries with high incidence of MDR TB, especially in eastern Europe (16).
Mathematical and epidemiologic models indicate that early diagnosis, effective treatment, and improved access to laboratory infrastructure could have a strong effect on the incidence of MDR TB in high-prevalence regions (17). However, few of such programmatic requirements are met at many sites in Europe at the present time (18).
Possible active transmission of strains causing MDR TB, as reflected by the large proportion of patients never having received TB treatment before in this European cohort, is consistent with recently reported data and deserves attention. A drug resistance survey conducted in Belarus in 2011 showed that 32.3% of new TB infections and 75.6% of previously treated TB infections had an MDR strain of M. tuberculosis (19). In Moldova, for which adequate surveillance data are available, 23.7% of new TB cases involve an MDR strain (3). A recent report of surveillance data in countries with >700 estimated MDR TB cases per year indicated that more than half of the reported pulmonary MDR TB cases were new cases (20).
More than 90% of strains from MDR TB patients had undergone DST for ≥1 second-line injectable drug and fluoroquinolone. The role of ethambutol and pyrazinamide for treatment of MDR TB is unclear. In our cohort, 97.6% and 45.0% of MDR TB strains were tested for resistance to ethambutol and pyrazinamide, respectively. In countries with a high incidence of TB, only 5.2% of MDR TB cases were tested for pyrazinamide resistance because of limited availability of liquid culture methods and special pH media requirements for pyrazinamide DST. Less than half of the strains tested were susceptible to these drugs. Currently, the mechanism of action of pyrazinamide in combination therapy and the relevance of in vitro DST for pyrazinamide are uncertain. Findings from this study raise questions about a universal recommendation to treat MDR TB with pyrazinamide throughout the entire course of treatment (21).
In our study cohort, 1 of 3 M. tuberculosis strains with resistance to at least rifampin and isoniazid were also resistant to protionamide/ethionamide, 1 of 4 were resistant to any second-line injectable drug, and 1 of 5 were resistant to a fluoroquinolone. Of all MDR TB cases, 6.8% fulfilled the definition of XDR TB. Surveillance data from the European Centre for Disease Prevention and Control indicated that 9.1% of cases of XDR TB in patients with MDR TB underwent second-line DST. Given the high proportion of strains that received a second-line DST, it is unlikely that these percentages are overstated because of preferred testing of patients at high risk for acquiring TB.
Our results are consistent with those from the Preserving Effective TB Treatment Study (PETTS) (22), which investigated second-line drug resistance in strains from 1,278 patients in 8 countries, including Latvia and Estonia, which were countries with study sites in this cohort. The main difference between PETTS study and ours was a high frequency of M. tuberculosis resistance to prothionamide/ethionamide in our cohort, which reflected the relatively higher frequency of treatment with this drug combination in eastern Europe than in other parts of the world (23). Recently published data from the PETTS study showed an increased risk of acquiring resistance to second-line drugs during treatment and increased baseline resistance (24). Increased resistance to second-line drugs is associated with higher proportions of treatment failures (6). It can be assumed, if one considers the findings from the PETTS study, that many of the patients in our cohort are at high risk for treatment failure.
Of particular concern is resistance to fluoroquinolones because these drugs are the core of new treatment regimens (25,26), including regimens for patients with drug-susceptible strains of M. tuberculosis (26). In our study, the capacity to perform DST for later-generation fluoroquinolones (levofloxacin and moxifloxacin) was only present for 19.2% of strains for levofloxacin and 8.4% of strains for moxifloxacin. Later generations of fluoroquinolones may still be effective for treatment of MDR TB in some patients when drug resistance to ofloxacin is documented (27). The capacity to perform DST for later generations of fluoroquinolones needs to be improved in the region.
Multivariable analysis showed that previous TB treatment and patient age <45 years showed an association with MDR TB (male sex and current homelessness showed an association in a weighted model). However, none of the other traditional risk factors for drug-resistant TB, such as HIV infection or birth in a foreign country (12), showed this association. Although previous treatment for TB and contact with persons infected with drug-resistant strains have been reported as strong risk factors for MDR TB, the role of HIV infection, young age, sex, and previous imprisonment are less clear (12,28). The high proportion of new cases and the lack of association of other traditional risk factors with drug-resistant TB suggest an increased role of ongoing transmission in the community outside established risk groups for becoming infected with drug-resistant strains of M. tuberculosis (19,20,29).
Our study has several major limitations. First, baseline data were obtained from an observational cohort study and were not derived from routine surveillance. Only 14 of 28 countries from the European Union and 2 countries outside the European Union were represented in the study. Site selection was based on voluntary participation in the study and being a center for the management of MDR TB. Because a high number of patients in Europe are being treated outside such centers, the generalizability of data might be limited. However, the included centers adhered to national policies regarding diagnosis and treatment of MDR TB patients and therefore reflect current practice. To provide a better estimation of representativeness of data for the situation in Europe, we additionally performed weighted analyses based on the sampling fraction and the expected number of reported MDR TB patients in the countries from which patients were recruited (online Technical Appendix). Results suggest that frequencies of drug resistance to second-line drugs might be underestimated by our analysis.
Second, some data collected were self-reported by patients and are prone to information bias. This limitation particularly applies to information on previous TB treatment in foreign-born patients, who might fear stigmatization in the country where treatment was provided.
Third, DST was performed at laboratories that used external quality control practices. However, quality control for testing of second-line drugs varies among sites and respective laboratories (30). Incompleteness of DST data for second-line drugs demonstrates the situation with which clinicians are confronted in making their management decisions and shows the need for scale up in laboratory testing, even in MDR TB reference centers in Europe.
Despite these limitations, our study identified 3 major concerns regarding TB in Europe. First, transmission of MDR strains of M. tuberculosis is ongoing. Second, diagnostic capacity is poor, especially for DST. Third, levels of resistance to second-line TB drugs are high. These factors must be addressed in any TB surveillance and control programs that are implemented.
This study (TBNET #30) was supported by the European Commission Seventh Framework Programme (FP7/2007-2013) under grant agreement FP7-223681 and was part of the EU FP7–funded TBPANNET project (http://www.tbpannet.org). C.L. is supported by the German Center for Infection Research.
Dr. Günther is a consultant physician and project manager of the TBNET MDR TB cohort at the Research Center Borstel, Borstel, Germany. His primary research interest is drug-resistant tuberculosis.
Acknowledgments
We thank Cordula Ehlers for providing excellent assistance.
Additional contributors from TBNET: Marcel Rowhani (Vienna, Austria); Vera Avchinko, Dzimitry Katovich, Dzimitri Klimuk, Valentina Lobik, Zoya Rohaya, Alexander Shirochyn (Minsk, Belarus); Jana Kotrbova, Martina Vasakova (Prague, Czech Republic); Aase Bengard Andersen (Copenhagen, Denmark); Nelleke Smitsman (Borstel, Germany); Ralf Mütterlein (Parsberg, Germany); Saverio de Lorenzo (Sondalo, Italy); Liga Rusmane (Riga, Latvia); Ana Donica, Ilie Cernenco (Chisinau, Moldavia); Vera Dubceac (Balti, Moldavia); Femke Cuppen, Inge de Guchtenaire (Nijmegen, the Netherlands); Robert Meesters, Mark te Pas, Bram Prins (Amsterdam, the Netherlands); Ana Atunes (Villa Nova de Gaia, Portugal); Dan Gainaru, Elmira Ibraim, Mirela Tigau (Bucharest, Romania); Juan Cayla, Laia Fina, Maria Luiza de Souza Galvao, José Maldonado (Barcelona, Spain).
References
- World Health Organization. Global tuberculosis report 2013. Geneva: The Organization; 2013.
- World Health Organization. WHO global task force outlines measures to combat XDR-TB worldwide. 2006 [cited 2014 May 10]. http://www.who.int/mediacentre/news/notes/2006/np29/en/
- European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Tuberculosis surveillance and monitoring in Europe 2014. Stockholm: The Centre; 2014.
- Grzybowski S, Enarson DA. The fate of cases of pulmonary tuberculosis under various treatment programmes. Bull Int Union Tuberc Lung Dis. 1978;53:70–5.
- Ahuja SD, Ashkin D, Avendano M, Banerjee R, Bauer M, Bayona JN, Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med. 2012;9:e1001300 . DOIPubMedGoogle Scholar
- Falzon D, Gandhi N, Migliori GB, Sotgiu G, Cox HS, Holtz TH, Resistance to fluoroquinolones and second-line injectable drugs: impact on multidrug-resistant TB outcomes. Eur Respir J. 2013;42:156–68. DOIPubMedGoogle Scholar
- van der Werf MJ, Kodmön C, Hollo V, Sandgren A, Zucs P. Drug resistance among tuberculosis cases in the European Union and European Economic Area, 2007 to 2012. Euro Surveill. 2014;19:20733 .PubMedGoogle Scholar
- van der Werf MJ, Langendam MW, Huitric E, Manissero D. Knowledge of tuberculosis-treatment prescription of health workers: a systematic review. Eur Respir J. 2012;39:1248–55. DOIPubMedGoogle Scholar
- Giehl C, Lange C, Duarte R, Bothamley G, Gerlach C, Cirillo DM, TBNET: collaborative research on tuberculosis in Europe. Eur J Microbiol Immunol (Bp). 2012;2:264–74.
- European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Tuberculosis surveillance and monitoring in Europe 2013. Stockholm: The Centre; 2013.
- World Health Organization. Definitions and reporting framework for tuberculosis—2013 revision [cited 2014 May 20]. http://apps.who.int/iris/bitstream/10665/79199/1/9789241505345_eng.pdf
- Lange C, Abubakr I, Alfenaar JW, Bothamley G, Caminero JA, Calvarho AC, Management of patients with multidrugresistant/extensively drug-resistant tuberculosis in Europe: a TBNET consensus statement. Eur Respir J. 2014;44:23–63. DOIPubMedGoogle Scholar
- Kreuter F, Valliant R. A survey on survey statistics: what is done and can be done in Stata. Stata J. 2007;7:1–21.
- Lemeshow S, Letenneur L, Dartigues JF, Lafont S, Orgogozo JM, Commenges D. Illustration of analysis taking into account complex survey considerations: the association between wine consumption and dementia in the PAQUID study. Am J Epidemiol. 1998;148:298–306 . DOIPubMedGoogle Scholar
- van Leth F, Kalisvaart NA, Erkens CG, Borgdoff MW. Projection of the number of patients with tuberculosis in the Netherlands in 2030. Eur J Public Health. 2009;19:424–7. DOIPubMedGoogle Scholar
- World Health Organization. Office for Europe: roadmap to prevent and combat drug-resistant tuberculosis, 2011 [cited 2014 Sep 18]. http://www.euro.who.int/en/publications/abstracts/roadmap-to-prevent-and-combat-drug-resistant-tuberculosis
- Uys PW, Warren R, van Helden PD, Murray M, Victor TC. Potential of rapid diagnosis for controlling drug-susceptible and drug-resistant tuberculosis in communities where Mycobacterium tuberculosis infections are highly prevalent. J Clin Microbiol. 2009;47:1484–90. DOIPubMedGoogle Scholar
- van der Werf MJ, Langendam MW, Huitric E, Manissero D. Multidrug resistance after inappropriate tuberculosis treatment: a meta-analysis. Eur Respir J. 2012;39:1511–9. DOIPubMedGoogle Scholar
- Skrahina A, Hurevich H, Zalutskaya A, Sahalchyk E, Astrauko A, Hoffner S, Multidrug-resistant tuberculosis in Belarus: the size of the problem and associated risk factors. Bull World Health Organ. 2013;91:36–45. DOIPubMedGoogle Scholar
- Royce S, Falzon D, van Weezenbeek C, Dara M, Hyder K, Hopewell P, Multidrug resistance in new tuberculosis patients: burden and implications. Int J Tuberc Lung Dis. 2013;17:511–3. DOIPubMedGoogle Scholar
- World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis. 2011 update. WHO/HTM/TB/2011.6:1-33 [cited 2014 Nov 13]. http://whqlibdoc.who.int/publications/2011/9789241501583_eng.pdf
- Dalton T, Cegielski P, Akksilp S, Asencios L, Campos Caoili J, Cho SN, Prevalence of and risk factors for resistance to second-line drugs in people with multidrug-resistant tuberculosis in eight countries: a prospective cohort study. Lancet. 2012;380:1406–17. DOIPubMedGoogle Scholar
- Hoffner S. Unexpected high levels of multidrug-resistant tuberculosis present new challenges for tuberculosis control. Lancet. 2012;380:1367–9. DOIPubMedGoogle Scholar
- Cegielski JP, Dalton T, Yagui M, Wattanaamornkiet W, Volchenkov GV, Via LE, Extensive drug resistance acquired during treatment of multidrug-resistant tuberculosis. Clin Infect Dis. 2014;59:1049–63. DOIPubMedGoogle Scholar
- Van Deun A, Kya Jai Maug A, Halim MA, Kumar Das P, Ranjan Sarker M, Daru P, Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2010;182:684–92. DOIPubMedGoogle Scholar
- Singh KP, Brown M, Murphy ME, Gillespie SH. Moxifloxacin for tuberculosis. Lancet Infect Dis. 2012;12:176, author reply 177–8 . DOIPubMedGoogle Scholar
- Jacobson KR, Tierney DB, Jeon CY, Mitnick CD, Murray MB. Treatment outcomes among patients with extensively drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis. 2010;51:6–14. DOIPubMedGoogle Scholar
- Kliiman K, Altraja A. Predictors of extensively drug-resistant pulmonary tuberculosis. Ann Intern Med. 2009;150:766–75. DOIPubMedGoogle Scholar
- Zhao Y, Xu S, Wang L, Chin DP, Wang S, Jiang G, National survey of drug-resistant tuberculosis in China. N Engl J Med. 2012;366:2161–70. DOIPubMedGoogle Scholar
- Hillemann D, Hoffner S, Cirillo D, Drobniewski F, Richter E, Rusch-Gerdes S. First evaluation after implementation of a quality control system for the second line drug susceptibility testing of Mycobacterium tuberculosis joint efforts in low and high incidence countries. PLoS ONE. 2013;8:e76765. DOIPubMedGoogle Scholar
Figure
Tables
Cite This Article1Additional contributors from TBNET are listed at the end of this article.
Table of Contents – Volume 21, Number 3—March 2015
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Christoph Lange, Clinical Tuberculosis Unit, Division of Clinical Infectious Diseases, German Center for Infection Research, Research Center Borstel, Parkallee 35, 23845 Borstel, Germany
Top