Volume 22, Number 1—January 2016
Letter
Highly Pathogenic Avian Influenza Virus, Midwestern United States
To the Editor: Novel highly pathogenic avian influenza (HPAI) viruses of subtypes H5N2, H5N8, and H5N1 have recently caused numerous outbreaks in commercial poultry farms in the United States and Canada (1). Risk for zoonotic transmission is low; humans are affected primarily from the extensive economic repercussions of suspending poultry-farming activities (1).
Large-scale research is under way, including case-control studies of infections on poultry farms and modeling studies to investigate the spread of virus in waterfowl (1,2). The US Department of Agriculture has published a report that summarizes various biosecurity measures of affected farms, results of airborne pathogen testing, and geospatial analyses correlating wind speed and direction to outbreaks (1). These studies found insufficient evidence to support any particular modes of virus spread and suggest that farms are contaminated from infected migrating waterfowl and/or unauthorized movements (e.g., of vehicles, equipment, persons, or animals) between farms and that unusually high wind speeds are the likely mechanism of spread (1). The spread from farm to farm, but not from barn to barn within a single farm (3), further adds to the puzzle of how infection has been transmitted.
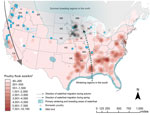
To better understand the outbreak behavior, we used publicly available sources (4–6) to create maps of outbreaks of HPAI virus, subtype H5, infections in relation to poultry distribution and wild bird migratory patterns (Figure; Technical Appendix Figures 1, 2; Video). From November 30, 2014, through June 17, 2015, a total of 280 outbreaks caused by HPAI virus subtype H5 in Canada and the United States were reported to the World Organisation for Animal Health (4). Most outbreaks occurred during April (n = 116) in commercial turkey farms (n = 154) and were caused by HPAI virus subtype H5N2 (n = 256) (Technical Appendix Figure 3). Related reassortant HPAI subtypes H5N8 and H5N1 were also found among infected poultry; however, these appeared infrequently. Subtype H5N1 appeared in 4 of 21 outbreaks in backyard and commercial farms and was found in 1 of 3 infections in a backyard farm. Backyard farms generally contain flocks for local consumption and implement fewer biosecurity measures (4).
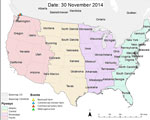
Initial outbreaks on poultry farms that began in November 2014, near the British Columbia–Washington State border, have been associated with timing of waterfowl migration and reported infection in migratory waterfowl (7,8). Subsequent surveillance of avian influenza virus in wild birds in the Pacific flyway has also shown sporadic infections caused by HPAI virus subtype H5, primarily in waterfowl species of the family Anseriformes (4) (Technical Appendix Table 1).
In late February 2015, however, HPAI virus subtype H5, emerged in US midwestern states, leading to a substantial number of outbreaks in commercial poultry farms in the region. The spread from west to east does not correlate with the direction of typical waterfowl migration, in which movement occurs from south to north. Unlike the earlier outbreaks in poultry in Canada, in the outbreaks in midwestern states, corresponding high numbers of virus were not detected in samples of wild birds in surrounding regions (despite increased surveillance). Of 3,300 samples tested, 1 sample tested positive for HPAI virus subtype H5 (4,9). In addition, most poultry farms were affected in April, and migratory waterfowl typically appear in Minnesota in March and April (Technical Appendix Figure 1). This February introduction of virus to Minnesota may be explained by an earlier-than-usual spring (10).
Minnesota and Iowa lie within regions where migrating waterfowl spend their breeding season, and waterfowl densities on commercial poultry farms are particularly high (Technical Appendix Figure 2). In southern parts of the United States, where poultry density is also high, isolated outbreaks of HPAI have occurred in poultry, although the introduction of virus into these regions did not result in a surge of outbreaks. The timing of waterfowl migration enables the mixing of highly dense populations of wild waterfowl and poultry, which likely plays a key role in spreading virus onto farms.
Of particular note, outbreaks in poultry were densely concentrated within Minnesota and Iowa in a spatial pattern inconsistent with the much more geographically dispersed spread of infection in wild birds. The magnitude and clustered distribution of poultry outbreaks are suggestive of local spread, rather than multiple introductions from passing migratory waterfowl. Genetic analyses have similarly shown evidence for concurrent multiple introductions as well as common source exposures, and surveys of affected farms have shown that local spread could be facilitated by the sharing of equipment by multiple farms or through animals entering barns (1).
The combination of high poultry densities and timing of waterfowl migration have likely predisposed Minnesota and Iowa to outbreaks of avian influenza among poultry flocks. However, consistent with US Department of Agriculture findings, local factors have likely also contributed to the large number of outbreaks in these states. We suggest that network modeling analyses would be valuable in exploring how virus may spread from farm to farm.
Acknowledgment
This study was funded by the National Health and Medical Research Council, project grant no. APP1082524. The contents of the published material are solely the responsibility of the individual authors and do not reflect the views of the National Health and Medical Research Council.
References
- United States Department of Agriculture, Animal and Plant Health Inspection Service. Epidemiological and other analyses of HPAI affected poultry flocks: June 15, 2015 report [cited 2015 Jul 7]. http://www.aphis.usda.gov/animal_health/animal_dis_spec/poultry/downloads/Epidemiologic-Analysis-June-15-2015.pdf
- United States department of Agriculture. Surveillance plan for highly pathogenic avian influenza in waterfowl in the United States, June 2015 [cited 2015 Jul 6]. http://www.aphis.usda.gov/animal_health/downloads/animal_diseases/ai/2015-hpai-surveillance-plan.pdf
- Center for Infectious Disease Research and Policy. Role of wild birds in US H5N2 outbreaks questioned [cited 2015 May 15]. http://www.cidrap.umn.edu/news-perspective/2015/03/role-wild-birds-us-h5n2-outbreaks-questioned
- World Organization of Animal Health (OIE). OIE reports. 2015 [cited 2015 Jul]. http://www.oie.int/en/animal-health-in-the-world/update-on-avian-influenza/2014/
- Bruhn MC, Munoz B, Cajka J, Smith G, Curry RJ, Wagener DK, Synthesized population databases: a geospatial database of US poultry farms. RTI Press publication no. MR-0023-1201. Research Triangle Park (NC): RTI Press; 2012 [cited DATE]. http://www.rti.org/pubs/mr-0023-1201-bruhn.pdf http://dx.doi.org/10.3768/rtipress.2012.mr.0023.1201
- Johnsgard PA. Waterfowl of North America, rev. ed. Lincoln (NE): University of Nebraska; 2010 [cited 2015 Jul 7] http://digitalcommons.unl.edu/biosciwaterfowlna/1
- Pasick J, Berhane Y, Joseph T, Bowes V, Hisanaga T, Handel K, Reassortant highly pathogenic influenza A H5N2 virus containing gene segments related to Eurasian H5N8 in British Columbia, Canada. Sci Rep. 2014;5:9484.PubMedGoogle Scholar
- Ip HS, Torchetti MK, Crespo R, Kohrs P, DeBruyn P, Mansfield KG, Novel Eurasian highly pathogenic influenza A H5 viruses in wild birds, Washington, USA, 2014. Emerg Infect Dis. 2015;21:886–90. DOIPubMedGoogle Scholar
- Minnesota Department of Natural Resources. After initial testing, DNR researchers still investigating avian flu in wildlife. June 18, 2015 [cited 2015 Jul 7]. http://news.dnr.state.mn.us/2015/06/18/after-initial-testing-dnr-researchers-still-investigating-avian-flu-in-wildlife/
- Zimpfer NL, Rhodes WE, Silverman ED, Zimmerman GS, Richkus KD. Trends in duck breeding populations, 1955–2015. US Fish & Wildlife Service Division of Migratory Bird Management Administrative Report, July 2, 2015 [cited 2015 Jul 7]. http://flyways.us/sites/default/files/uploads/trends_in_duck_breeding_populations_2015.pdf
Figures
Cite This ArticleRelated Links
Table of Contents – Volume 22, Number 1—January 2016
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Chau Bui, School of Public Health and Community Medicine, Samuels Building F25, Kensington Campus, University of New South Wales, Sydney, Australia
Top