Volume 22, Number 12—December 2016
Letter
Unique Strain of Borrelia miyamotoi in Ixodes pacificus Ticks, California, USA
To the Editor: Borrelia miyamotoi causes a recently recognized tickborne zoonosis in Eurasia and North America (1). The species has been detected in Ixodes persulcatus ticks in Asia and Russia, I. ricinus ticks in Europe, and I. scapularis and I. pacificus ticks in North America. In most of these regions, B. miyamotoi is sympatric with Lyme disease agents, such as B. burgdorferi, and both pathogens are transmitted locally by the same species of Ixodes ticks. B. miyamotoi generally is less prevalent than B. burgdorferi in nymphs and adults in North America (2), except in California, where the prevalences of the 2 species in populations of nymphal and adult I. pacificus ticks are similar (3–6).
Genomes of isolates of B. miyamotoi from I. persulcatus and I. scapularis ticks have been sequenced (7). Comparatively less was known about B. miyamotoi in I. pacificus ticks. Limited sequence data of 16S ribosomal RNA and flagellin genes and the 16S-23S intergenic spacer (IGS) were sufficient to identify the I. pacificus–borne spirochete as a sister taxon to B. miyamotoi from elsewhere (3,4). Until B. miyamotoi is isolated from I. pacificus ticks, determination of additional sequences from I. pacficus ticks from California addresses 2 issues of phylogeographic and potential epidemiologic importance: Is the California population of B. miyamotoi more akin to the strain across the Pacific Rim or to the strain thousands of kilometers to the east in North America? Will the noted pattern of exclusive association between the genotype of B. miyamotoi and the species of Ixodes vector continue to hold (1)?
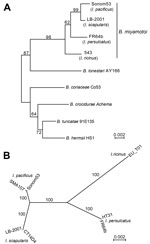
We evaluated DNA extracts of B. miyamotoi–infected I. pacificus ticks collected by and stored at 2 laboratories in the San Francisco Bay area of California. Ticks had been collected while questing either on low vegetation or in leaf litter. To confirm B. miyamotoi in candidate extracts and to exclude extracts that also contained B. burgdorferi sensu lato, we used a quantitative PCR, which differentiates relapsing fever and Lyme disease group species (2). Two extracts that met these criteria were Sonom53 from a nymph in Sonoma County, California (38.328758, −122.625286), and SMA107 from an adult male tick in San Mateo County, California (37.466999, −122.283532). We amplified DNA by PCR for 1,307 bp of the 16S ribosomal RNA gene (8) and variable lengths of the IGS (9). In addition, we performed PCR amplification and sequencing of partial sequences of 8 chromosomal genes used for multilocus sequence typing (MLST): clpA, clpX, nifS, pepX, pyrG, recG, rplB, and uvrA (10). The primers (and annealing temperatures for 35 cycles) were as given (http://pubmlst.org/borrelia), except for these modifications: clpA (53°C); clpX forward 5′-CCGTTGCTATTTGTTTTGAATGCTCT-3′ (55°C); pepX forward 5′-TTAAAACTTGATGATAAATGGTCATTA-3′ and reverse 5′-TTAAAACTTGATGATAAATGGTCATTA-3′ (52°C); pyrG forward 5′-CTTTTAGTAATTGAGATTGGTGGT-3′ and reverse 5′-CAGCATCAAGTATTCCACAAAC-3′ (55°C); recG forward 5′-CTAGCATTCCTTTAGTTGAGGC-3′ and reverse 5′-TTSTGTTAAAGGTTCCTTATAAAG-3′ (52°C); rplB forward 5′-ATTAAAACTTATAGGCCAAAAAC-3′ and reverse 5′-GGCTGACCCCAAGGAGAT-3′ (55°C); and uvrA forward 5′-GCTTAAATTTTTAATTGATGTTGGA-3′ and reverse 5′-CAAGGAACAAAAATRTCAGGC-3′ (52°C). On a Bio-Rad T100 thermal cycler (Hercules, CA, USA) and with Apex Master mix (Genesee Scientific, San Diego, CA, USA), PCR extension at 72°C was 1.5 min for clpX and 1.0 min for others; final elongation was for 5 min at 72°C. Products were sequenced over both strands at GENEWIZ (San Diego, CA, USA) by the Sanger method either directly or after cloning into a plasmid vector. Resultant sequences were aligned with homologous sequences (Figure). Alignments and distance neighbor-joining and maximum-likelihood phylograms were generated with Seaview4 (http://doua.prabi.fr/software/seaview). The equal length MLST sequences, as specified (10), for each locus were concatenated.
We determined a rooted neighbor-joining phylogram of 16S ribosomal RNA gene sequences of B. miyamotoi from different Ixodes species and that of Amblyomma americanum tickborne B. lonestari (Figure, panel A). Other species of the relapsing fever group served as an outgroup. B. miyamotoi sequences from I. pacificus ticks in 2 San Francisco Bay area counties clustered with sequences from I. scapularis–borne organisms rather than with I. persulcatus–borne organisms in Asia or an I. ricinus–borne isolate in Europe. This analysis confirmed that the organism in I. pacificus was B. miyamotoi. An unrooted phylogram of 4,776 nt of concatenated MLST sequences originating in I. pacificus, I. scapularis, I. persulcatus, or I. ricinus ticks had similar topology and differentiated the different strains (Figure, panel B). The B. miyamotoi organisms from 2 counties differed at 1 position, a synonymous transition in pyrG, among the MLST loci. IGS sequences of the 2 organisms were the same (GenBank accession no. KU184505) and identical to the IGS of other B. miyamotoi in I. pacificus ticks (e.g., GenBank accession no. KF957669). As observed previously (4,9), they were distinct from strains associated with other Ixodes species.
In conclusion, we identified differences in several genetic loci between B. miyamotoi in I. pacificus ticks and organism strains associated with other Ixodes species. However, we found a close phylogenetic relationship between organisms from the far-western and the northeastern United States.
Acknowledgments
We thank Parth Sitlani for technical assistance.
This research was supported in part by grant R21 AI100236 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, to A.G.B. and by a generous gift from Tom Eames and Geneva Anderson to R.S.L.
References
- Krause PJ, Fish D, Narasimhan S, Barbour AG. Borrelia miyamotoi infection in nature and in humans. Clin Microbiol Infect. 2015;21:631–9. DOIPubMedGoogle Scholar
- Barbour AG, Bunikis J, Travinsky B, Hoen AG, Diuk-Wasser MA, Fish D, et al. Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am J Trop Med Hyg. 2009;81:1120–31. DOIPubMedGoogle Scholar
- Mun J, Eisen RJ, Eisen L, Lane RS. Detection of a Borrelia miyamotoi sensu lato relapsing-fever group spirochete from Ixodes pacificus in California. J Med Entomol. 2006;43:120–3. DOIPubMedGoogle Scholar
- Fedorova N, Kleinjan JE, James D, Hui LT, Peeters H, Lane RS. Remarkable diversity of tick or mammalian-associated borreliae in the metropolitan San Francisco Bay area, California. Ticks Tick Borne Dis. 2014;5:951–61. DOIPubMedGoogle Scholar
- Padgett K, Bonilla D, Kjemtrup A, Vilcins IM, Yoshimizu MH, Hui L, et al. Large scale spatial risk and comparative prevalence of Borrelia miyamotoi and Borrelia burgdorferi sensu lato in Ixodes pacificus. PLoS One. 2014;9:e110853. DOIPubMedGoogle Scholar
- Salkeld DJ, Nieto NC, Carbajales-Dale P, Carbajales-Dale M, Cinkovich SS, Lambin EF. Disease risk and landscape attributes of tick-borne Borrelia pathogens in the San Francisco Bay area, California. PLoS One. 2015;10:e0134812. DOIPubMedGoogle Scholar
- Barbour AG. Phylogeny of a relapsing fever Borrelia species transmitted by the hard tick Ixodes scapularis. Infect Genet Evol. 2014;27:551–8. DOIPubMedGoogle Scholar
- Barbour AG, Maupin GO, Teltow GJ, Carter CJ, Piesman J. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease–like illness. J Infect Dis. 1996;173:403–9. DOIPubMedGoogle Scholar
- Bunikis J, Tsao J, Garpmo U, Berglund J, Fish D, Barbour AG. Typing of Borrelia relapsing fever group strains. Emerg Infect Dis. 2004;10:1661–4. DOIPubMedGoogle Scholar
- Margos G, Gatewood AG, Aanensen DM, Hanincova K, Terekhova D, Vollmer SA, et al. MLST of housekeeping genes captures geographic population structure and suggests a European origin of Borrelia burgdorferi. Proc Natl Acad Sci U S A. 2008;105:8730–5. DOIPubMedGoogle Scholar
Figure
Cite This ArticleRelated Links
Table of Contents – Volume 22, Number 12—December 2016
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Alan G. Barbour, Department of Microbiology and Molecular Genetics, University of California Irvine, 843 Health Sciences Rd., Irvine, CA 92697-4028, USA
Top