Volume 23, Number 3—March 2017
Research Letter
Molecular Verification of New World Mansonella perstans Parasitemias
Cite This Article
Citation for Media
Abstract
We obtained ribosomal and mitochondrial DNA sequences from residents of Amazonas state, Brazil, with Mansonella parasitemias. Phylogenetic analysis of these sequences confirm that M. ozzardi and M. perstans parasites occur in sympatry and reveal the close relationship between M. perstans in Africa and Brazil, providing insights into the parasite’s New World origins.
Mansonella perstans is one of the most prevalent and poorly understood parasites known to cause parasitemias in humans (1–3). An estimated 114 million persons are infected with M. perstans parasites in Africa alone, and M. perstans parasitemias have also been repeatedly reported to occur in continental South America (1,2). In Uganda, M. perstans infections and parasitic loads have been shown to map closely with the larval breeding sites of its known vector, the Culicoides midge (1). Almost nothing is known about the parasites’ epidemiology in continental South America; however, it has been established that simuliids and a diverse range of Ceratopogonid vector species transmit M. ozzardi parasites in Latin America (1). Thus, it cannot safely assumed that the epidemiology of M. perstans in Latin America is particularly similar to its epidemiology in Africa (1,2).
Like most reports of M. perstans in Africa, reports of the occurrence of M. perstans in South America have almost always been based on morphologically identified microfilariae observed in blood smears (1,2). However, in contrast to the situation in Africa, where only 1 parasitemia-causing Mansonella parasite occurs, reports of M. perstans in South America have been limited to equatorial rainforest regions, where other Mansonella parasitemia-causing parasites also commonly occur (1–4). Therefore, microscopy-based Mansonella parasitemia diagnoses in Latin America can be regarded as more prone to error than those made in Africa (1–6). Conspicuously, M. perstans DNA sequences originating outside of Africa have until now been missing, and the relationship between M. perstans in Africa and M. perstans in the New World has been a mystery (1).
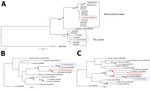
By using 3 DNA sequences commonly used in the molecular systematics of filarial parasites (the nuclear internal transcribed spacer 1 [ITS1]–based ribosomal DNA sequence [7] and the mitochondrial 12S and cytochrome c oxidase subunit 1 genes [6]), we confirmed M. perstans microfilariae morphologic identifications made using thick blood smears prepared from persons residing in the village of São Gabriel da Cachoeira, Amazonas state, Brazil. Besides providing verification of M. perstans morphologic identifications, the ITS1 sequences generated for this study allowed a phylogenetic analysis with M. perstans from Africa. The ribosomal ITS1 M. perstans from Brazil clustered with other M. perstans ITS1 sequences originating from Africa in a strongly (>94%) bootstrap-supported M. perstans–exclusive monophyletic group (Figure). Similarly, M. ozzardi ITS1 sequences obtained from parasites from Brazil clustered in another strongly (>97%) bootstrap-supported monophyletic group containing only M. ozzardi origin sequences.
The genetic distance between the ITS1 sequences of M. perstans from Brazil and their closest relatives from Africa is very small (corresponding to <1% divergence across 396 nucleotide positions). From the ITS1-based phylogenetic analysis, the M. perstans from Brazil appear to be more closely related to some M. perstans in Africa than they are to others. The ITS1 sequences from M. perstans previously described as M. perstans “deux” (8) and originating from Gabon can be observed in a bootstrap-supported cluster forming a sister clade to the bootstrap-supported monophyletic cluster containing the M. perstans from Brazil, which also contains sequences originating from Cameroon, Côte d’Ivoire, Equatorial Guinea, Gabon, Mali, and Sierra Leone. Thus, our results suggest that M. perstans arrived in Latin America after the standard form of M. perstans diverged from the M. perstans “deux” form.
Sequences from mitochondrial genes 12S rDNA and cytochrome c oxidase subunit 1 have also been recovered from blood samples in Brazil and used to confirm morphologic and ITS1-based Mansonella parasite identifications (6). Phylogenetic analysis performed with these mitochondrial gene segments was consistent with our ITS1 analysis and also suggest that M. perstans arrived in Latin America very recently (Figure). In addition to verifying that South America does indeed have the conditions to support M. perstans and providing a useful reference for vector incrimination and other epidemiologic studies, our findings have also provided insights into the origin of the M. perstans parasite in South America. Given how similar our findings are to those obtained when Onchocerca volvulus parasite mitogenomes from Latin America and Africa have been compared, they suggest that M. perstans, like O. volvulus, probably arrived in Latin America as a consequence of the slave trade (9–10).
Ms. Tavares da Silva is a master’s degree student at Instituto Leônidas e Maria Deane of Fundação Oswaldo Cruz. Her primary research interest is the epidemiology of Mansonella parasitism in the Amazon region of Brazil.
Acknowledgment
The work presented in this study was performed as part of a study called “Mansonelose em área urbana de São Gabriel da Cachoeira, Amazonas,” which received ethical clearance from the Comité de Ética em Pesquisa do Instituto Oswaldo Cruz (CAAE: 41678515.1.0000.5248) and financial support from the Fundação de Amparo à Ciência e Pesquisa of Amazonas state (processo 062.00647/2014) and Programa Pesquisa Sistema Único de Saúde and Programa de Excelência em Pesquisa Básica e Aplicada em Saúde project grants (processo 062.02005/2014) awarded by the Fundação de Amparo à Pesquisa do Estado do Amazonas.
References
- Medeiros JF, Crainey JL, Pessoa FA, Luz SL. Mansonelliasis. In: Marcondes CB, editor. Arthropod borne diseases. Cham (Switzerland): Springer International Publishing; 2017. p. 562.
- Simonsen PE, Onapa AW, Asio SM. Mansonella perstans filariasis in Africa. Acta Trop. 2011;120(Suppl 1):S109–20. DOIPubMedGoogle Scholar
- Phillips RO, Frimpong M, Sarfo FS, Kretschmer B, Beissner M, Debrah A, et al. Infection with Mansonella perstans nematodes in Buruli ulcer patients, Ghana. Emerg Infect Dis. 2014;20:1000–3. DOIPubMedGoogle Scholar
- Post RJ, Adams Z, Shelley AJ, Maia-Herzog M, Luna Dias AP, Coscarón S. The morphological discrimination of microfilariae of Onchocerca volvulus from Mansonella ozzardi. Parasitology. 2003;127:21–7. DOIPubMedGoogle Scholar
- Bain O, Otranto D, Diniz DG, dos Santos JN, de Oliveira NP, Frota de Almeida IN, et al. Human intraocular filariasis caused by Pelecitus sp. nematode, Brazil. Emerg Infect Dis. 2011;17:867–9. DOIPubMedGoogle Scholar
- Marcos LA, Arrospide N, Recuenco S, Cabezas C, Weil GJ, Fischer PU. Genetic characterization of atypical Mansonella (Mansonella) ozzardi microfilariae in human blood samples from northeastern Peru. Am J Trop Med Hyg. 2012;87:491–4. DOIPubMedGoogle Scholar
- Tang TH, López-Vélez R, Lanza M, Shelley AJ, Rubio JM, Luz SL. Nested PCR to detect and distinguish the sympatric filarial species Onchocerca volvulus, Mansonella ozzardi and Mansonella perstans in the Amazon Region. Mem Inst Oswaldo Cruz. 2010;105:823–8. DOIPubMedGoogle Scholar
- Mourembou G, Fenollar F, Lekana-Douki JB, Ndjoyi Mbiguino A, Maghendji Nzondo S, Matsiegui PB, et al. Mansonella, including a potential new species, as common parasites in children in Gabon. PLoS Negl Trop Dis. 2015;9:e0004155. DOIPubMedGoogle Scholar
- Crainey JL, Medeiros JF, Pessoa FA, Luz SL. Onchocerciasis. In: Marcondes CB, editor. Arthropod borne diseases. Cham (Switzerland): Springer International Publishing; 2017. p. 562.
- Crainey JL, Silva TR, Encinas F, Marín MA, Vicente AC, Luz SL. The mitogenome of Onchocerca volvulus from the Brazilian Amazonia focus. Mem Inst Oswaldo Cruz. 2016;111:79–81. DOIPubMedGoogle Scholar
Figure
Cite This Article1These authors contributed equally to this article.
Table of Contents – Volume 23, Number 3—March 2017
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
James Lee Crainey, Laboratório de Ecologia de Doenças Transmissíveis na Amazônia (EDTA), Instituto Leônidas e Maria Deane (Fiocruz), Rua Teresina, 476, Adrianópolis, Manaus, Amazonas, CEP 69027-070, Brazil
Top