Volume 23, Number 6—June 2017
Research Letter
Angiostrongylus cantonensis Meningitis and Myelitis, Texas, USA
Cite This Article
Citation for Media
Abstract
Infection with Angiostrongylus cantonensis roundworms is endemic in Southeast Asia and the Pacific Basin. A. cantonensis meningitis and myelitis occurred in summer 2013 in a child with no history of travel outside of Texas, USA. Angiostrongyliasis is an emerging neurotropic helminthic disease in Texas and warrants increased awareness among healthcare providers.
In summer 2013, a previously healthy Caucasian 12-month-old girl was brought for treatment to a children’s hospital in Houston, Texas, USA, on the 11th day of illness (day 11), manifesting intermittent fever, lethargy, and emesis. She had been evaluated by a pediatrician on day 3 and diagnosed with presumed viral infection. She attended day care, had no history of sick contacts, and apart from dogs in the house, had no notable other exposures.
At hospital admission, physical examination showed vital signs within reference ranges, mild distress, lethargy, and irritability with no focal deficits or signs of meningeal irritation. Blood test results showed leukocytosis (17,900 cells/mm3 with 20% eosinophils). Cerebrospinal fluid (CSF) examination showed 8 erythrocytes and 568 leukocytes/mm3 with 26% eosinophils. Results of bacterial cultures and PCR of CSF for herpes simplex virus and enterovirus were negative. She had no serologic evidence of acute infection with West Nile virus or HIV. Magnetic resonance imaging (MRI) of the brain showed normal results. She received ceftriaxone, vancomycin, and acyclovir from days 11 through 15 with no clinical improvement.
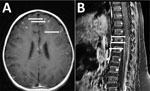
On day 16, because the child had been exposed to dogs, she was empirically treated for presumed Toxocara infection with albendazole and prednisone for 5 days. Her clinical condition improved. However, 2 days after she completed the albendazole and prednisone regimen (day 23), fever and lethargy recurred, she was unable to bear weight, and CSF analysis showed increased eosinophilia (1,500 leukocytes/mm3 with 35% eosinophils). On day 23, MRI showed diffuse leptomeningeal enhancement in the brain and spinal cord, diffuse punctate areas of cortical infarction, and intramedullary enhancement (Figure). On the same day, she was again treated with albendazole and prednisone with good response.
Results of serologic testing performed at the hospital were negative for Trichinella, Toxocara, and Schistosoma spp. Serum and CSF samples were sent to the Centers for Diseases Control and Prevention (Atlanta, GA, USA); PCR results showed CSF was positive for Angiostrongylus cantonensis, and serum was positive for Toxocara Strongyloides stercoralis IgG. On day 31, we diagnosed A. cantonensis meningitis and myelitis with cross-reactions to S. stercoralis and Toxocara spp. The patient completed 2 weeks of albendazole therapy (from day 23 through day 37) and 3 months of prednisone therapy with slow tapering. The patient’s symptoms completely resolved by day 64. Lumbar punctures on days 35, 56, and 104 showed gradual return of CSF parameters to normal ranges. On day 56, results of an MRI of the brain were unremarkable.
The case was reported to the local health department, which tested snails trapped close to the patient’s residence. No evidence of A. cantonensis roundworms was found.
In 1987, A. cantonensis roundworm was identified for the first time in North America in rodents. (1). Although a PCR-based assay did not detect A. cantonensis roundworm in samples of intermediate hosts (snails) in Houston, Texas, the parasite has been repeatedly identified in southeastern and Gulf states (Louisiana, Oklahoma, Mississippi, Florida) (2,3). In parallel, human infections have increased. The first reported human case of A. cantonensis meningitis in a nontraveler in the contiguous United States occurred in 1995 in a 10-year-old boy in New Orleans, Louisiana, who had eaten raw snails (4). Since the case we report, 2 additional cases in children were reported in Houston, in the spring of 2016 (5).
The frequency of A. cantonensis infections in humans is likely underreported due to a combination of factors, including the frequent self-limited course of the infection, lack of awareness of the parasite, limited availability of diagnostic testing, and lack of national surveillance. Although the disease usually resolves spontaneously, case-fatality rates can reach 5% (6). Lack of clinical suspicion for angiostrongyliasis on the basis of signs and symptoms and delay in initiation of treatment can lead to neurologic deterioration, especially in young children, as demonstrated in our patient and another report (7).
In conclusion, angiostrongyliasis is an emerging neurotropic helminthic disease in Texas. Increased awareness of A. cantonensis infection in humans is needed among healthcare providers.
Dr. Al Hammoud is a pediatric infectious diseases fellow at University of Texas Health, McGovern Medical School. Her areas of interest include general pediatric infectious diseases, HIV-associated nephropathy, and immunization response in HIV-infected children.
References
- Campbell BG, Little MD. The finding of Angiostrongylus cantonensis in rats in New Orleans. Am J Trop Med Hyg. 1988;38:568–73.PubMedGoogle Scholar
- Teem JL, Qvarnstrom Y, Bishop HS, da Silva AJ, Carter J, White-McLean J, et al. The occurrence of the rat lungworm, Angiostrongylus cantonensis, in nonindigenous snails in the Gulf of Mexico region of the United States. Hawaii J Med Public Health. 2013;72(Suppl 2):11–4.PubMedGoogle Scholar
- York EM, Creecy JP, Lord WD, Caire W. Geographic range expansion for rat lungworm in North America. Emerg Infect Dis. 2015;21:1234–6. DOIPubMedGoogle Scholar
- New D, Little MD, Cross J. Angiostrongylus cantonensis infection from eating raw snails. N Engl J Med. 1995;332:1105–6. DOIPubMedGoogle Scholar
- Foster CE, Nicholson EG, Chun AC, Gharfeh M, Anvari S, Seeborg FO, et al. Angiostrongylus cantonensis infection: a cause of fever of unknown origin in pediatric patients. Clin Infect Dis. 2016;63:1475–8. DOIPubMedGoogle Scholar
- Tseng YT, Tsai HC, Sy CL, Lee SS, Wann SR, Wang YH, et al. Clinical manifestations of eosinophilic meningitis caused by Angiostrongylus cantonensis: 18 years’ experience in a medical center in southern Taiwan. J Microbiol Immunol Infect. 2011;44:382–9. DOIPubMedGoogle Scholar
- Evans-Gilbert T, Lindo JF, Henry S, Brown P, Christie CD. Severe eosinophilic meningitis owing to Angiostrongylus cantonensis in young Jamaican children: case report and literature review. Paediatr Int Child Health. 2014;34:148–52. DOIPubMedGoogle Scholar
Figure
Cite This ArticleTable of Contents – Volume 23, Number 6—June 2017
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Roukaya Al Hammoud, University of Texas Health, McGovern Medical School, Pediatric Infectious Diseases Division, 6431 Fannin St, Ste 3.126, Houston, TX 77030, USA
Top