Volume 25, Number 10—October 2019
Research
Comparison of Serologic Assays for Middle East Respiratory Syndrome Coronavirus
Figure 2
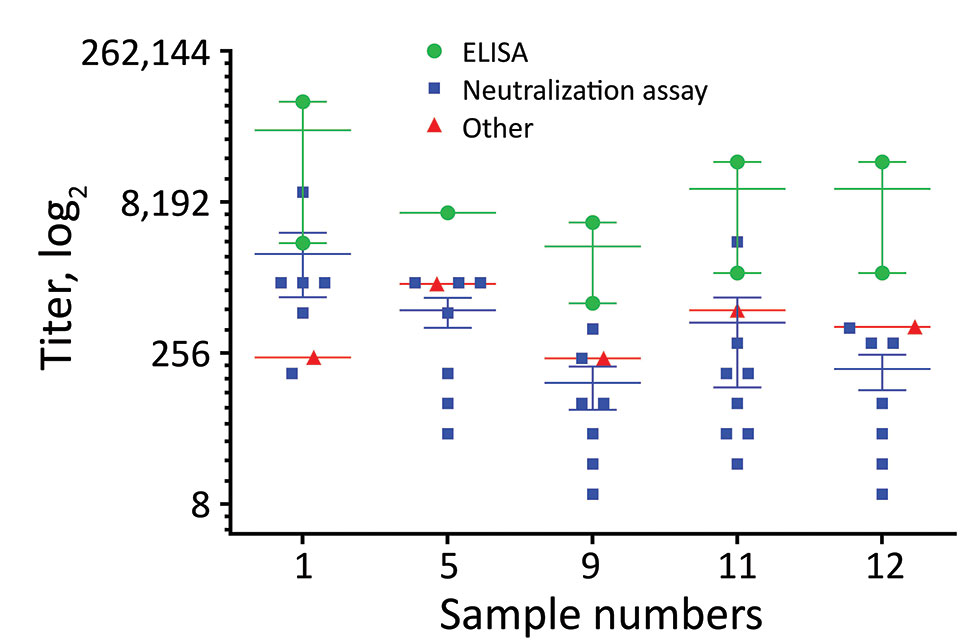
Figure 2. Endpoint titers of individual positive patient plasma samples in study of serologic assays for MERS-CoV. The titers for the 5 individual MERS-CoV–positive patient plasma were determined by ELISA (green circles), neutralization assays (blue squares), and other assays (red triangles). Horizontal lines indicate the mean for each assay type; error bars show SD between assays. MERS-CoV, Middle East respiratory syndrome coronavirus.
1Study participants who contributed data are listed at the end of this article.
Page created: September 17, 2019
Page updated: September 17, 2019
Page reviewed: September 17, 2019
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.