17DD Yellow Fever Revaccination and Heightened Long-Term Immunity in Populations of Disease-Endemic Areas, Brazil
Ana Carolina Campi-Azevedo

, Vanessa Peruhype-Magalhāes, Jordana Grazziela Coelho-dos-Reis, Lis Ribeiro Antonelli, Christiane Costa-Pereira, Elaine Speziali, Laise Rodrigues Reis, Jandira Aparecida Lemos, José Geraldo Leite Ribeiro, Luiz Antônio Bastos Camacho, Maria de Lourdes de Sousa Maia, Sheila Maria Barbosa de Lima, Marisol Simões, Reinaldo de Menezes Martins, Akira Homma, Luiz Cosme Cota Malaquias, Pedro Luiz Tauil, Pedro Fernando Costa Vasconcelos, Alessandro Pecego Martins Romano, Carla Magda Domingues, Andréa Teixeira-Carvalho, Olindo Assis Martins-Filho

, and Collaborative Group for Studies of Yellow Fever Vaccine
Author affiliations: Instituto René Rachou of Fundação Oswaldo Cruz (FIOCRUZ-Minas),; Belo Horizonte, Brazil (A.C. Campi-Azevedo, V. Peruhype-Magalhāes, J.G. Coelho-dos-Reis, L.R. Antonelli, C. Costa-Pereira, E. Speziali, L.R. Reis, A. Teixeira-Carvalho, O.A. Martins-Filho); Universidade Federal de Minas Gerais, Belo Horizonte (J.G. Coelho-dos-Reis); Secretaria Municipal de Saúde, Belo Horizonte (J.A. Lemos); Secretaria do Estado de Saúde de Minas Gerais, Belo Horizonte (J.G.L. Ribeiro); Escola Nacional de Saúde Pública (FIOCRUZ-Rio), Rio de Janeiro, Brazil (L.A.B. Camacho); Instituto de Tecnologia em Imunobiológicos Bio-Manguinhos (FIOCRUZ-Rio),; Rio de Janeiro (M. de Lourdes de Sousa Maia, S.M. Barbosa de Lima, M. Simões, R. de Menezes Martins, A. Homma); Universidade Federal de Alfenas, Alfenas, Brazil (L.C.C. Malaquias); Universidade de Brasília, Brasília, Brazil (P.L. Tauil); Instituto Evandro Chagas, Ananindeua, Brazil (P.F.C. Vasconcelos); Secretaria de Vigilância em Saúde—Ministério da Saúde, Brasília (A.P.M. Romano, C.M. Domingues)
Main Article
Figure 8
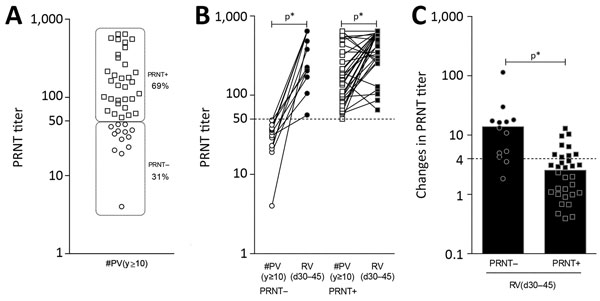
Figure 8. Baseline PRNT reactivity before revaccination (A) and impact on PRNT levels triggered by secondary 17DD vaccination for YF (B, C). Subgroups PRNT– (circles) and PRNT+ (squares) were defined considering the cutoff criterion for PRNT positivity at serum dilution >1:50. The ability of secondary vaccination to increase the levels of neutralizing antibodies as well as the magnitude of changes in PRNT titers (baseline fold changes) are indicated for PRNT– (filled circles) and PRNT+ (filled squares) vaccinees. Increases in PRNT titer by a factor of >4 at follow-up were considered as classical criteria to evaluate booster response. Bars indicate significant differences (p<0.05) between subgroups. Participant subgroups indicate number of days or years since vaccination (in parentheses). #PV, had primary vaccination >10 years previously; PRNT, plaque-reducing neutralization test; RV, revaccinated; YF, yellow fever.
Main Article
Page created: July 16, 2019
Page updated: July 16, 2019
Page reviewed: July 16, 2019
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
