17DD Yellow Fever Revaccination and Heightened Long-Term Immunity in Populations of Disease-Endemic Areas, Brazil
Ana Carolina Campi-Azevedo

, Vanessa Peruhype-Magalhāes, Jordana Grazziela Coelho-dos-Reis, Lis Ribeiro Antonelli, Christiane Costa-Pereira, Elaine Speziali, Laise Rodrigues Reis, Jandira Aparecida Lemos, José Geraldo Leite Ribeiro, Luiz Antônio Bastos Camacho, Maria de Lourdes de Sousa Maia, Sheila Maria Barbosa de Lima, Marisol Simões, Reinaldo de Menezes Martins, Akira Homma, Luiz Cosme Cota Malaquias, Pedro Luiz Tauil, Pedro Fernando Costa Vasconcelos, Alessandro Pecego Martins Romano, Carla Magda Domingues, Andréa Teixeira-Carvalho, Olindo Assis Martins-Filho

, and Collaborative Group for Studies of Yellow Fever Vaccine
Author affiliations: Instituto René Rachou of Fundação Oswaldo Cruz (FIOCRUZ-Minas),; Belo Horizonte, Brazil (A.C. Campi-Azevedo, V. Peruhype-Magalhāes, J.G. Coelho-dos-Reis, L.R. Antonelli, C. Costa-Pereira, E. Speziali, L.R. Reis, A. Teixeira-Carvalho, O.A. Martins-Filho); Universidade Federal de Minas Gerais, Belo Horizonte (J.G. Coelho-dos-Reis); Secretaria Municipal de Saúde, Belo Horizonte (J.A. Lemos); Secretaria do Estado de Saúde de Minas Gerais, Belo Horizonte (J.G.L. Ribeiro); Escola Nacional de Saúde Pública (FIOCRUZ-Rio), Rio de Janeiro, Brazil (L.A.B. Camacho); Instituto de Tecnologia em Imunobiológicos Bio-Manguinhos (FIOCRUZ-Rio),; Rio de Janeiro (M. de Lourdes de Sousa Maia, S.M. Barbosa de Lima, M. Simões, R. de Menezes Martins, A. Homma); Universidade Federal de Alfenas, Alfenas, Brazil (L.C.C. Malaquias); Universidade de Brasília, Brasília, Brazil (P.L. Tauil); Instituto Evandro Chagas, Ananindeua, Brazil (P.F.C. Vasconcelos); Secretaria de Vigilância em Saúde—Ministério da Saúde, Brasília (A.P.M. Romano, C.M. Domingues)
Main Article
Figure 7
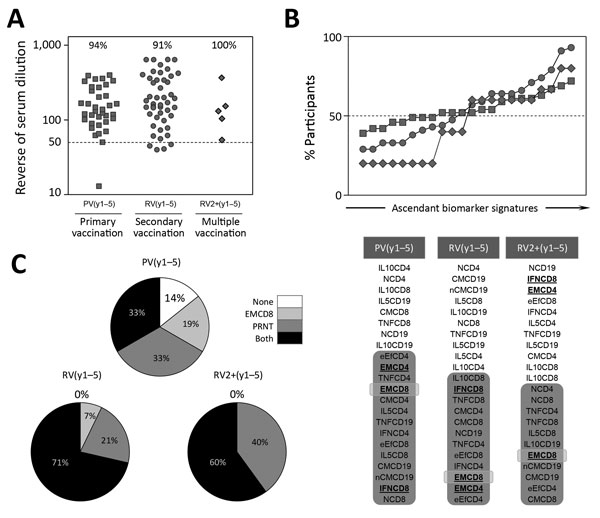
Figure 7. Overall profile of immune response after primary, secondary, or multiple 17DD vaccination for YF. A) Levels of 17DD-YF–specific neutralizing antibodies; B) 17DD-YF–specific phenotypic and functional biomarkers; and C) resultant memory status PRNT and EMCD8 measurement (PRNT and EMCD8) for individual participants. Results are expressed in reverse of serum dilution, percentage of participants with overlaid biomarker signatures, and resultant memory status at 1–5 years after primary (gray circle), secondary (gray square), or multiple (gray diamond) vaccination. Participant subgroups indicate number of days or years since vaccination (in parentheses; d0 for those never vaccinated). #PV, had primary vaccination >10 years previously; IFN, interferon; IL, interleukin; NV, not vaccinated; PRNT, plaque-reducing neutralization test; PV, had primary vaccination only; RV, revaccinated; TNF, tumor necrosis factor; YF, yellow fever.
Main Article
Page created: July 16, 2019
Page updated: July 16, 2019
Page reviewed: July 16, 2019
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
