Volume 26, Number 10—October 2020
Research Letter
Relative Bradycardia in Patients with Mild-to-Moderate Coronavirus Disease, Japan
Cite This Article
Citation for Media
Abstract
Coronavirus disease is reported to affect the cardiovascular system. We showed that relative bradycardia was a common characteristic for 54 patients with PCR-confirmed mild-to-moderate coronavirus disease in Japan. This clinical sign could help clinicians to diagnose this disease.
Pulse rate usually increases 18 beats/min for each 1°C increase in body temperature (1). However, in some specific infectious diseases, pulse rate does not increase as expected, a condition called relative bradycardia. High fever (temperature >39°C) for patients with coronavirus disease (COVID-19) has been reported (2,3), but the association between fever and pulse rate has not been investigated. We investigated relative bradycardia as a characteristic clinical feature in patients with mild-to-moderate COVID-19.
Retrospective analyses of routinely collected clinical records of COVID-19 patients were approved by the ethics committee of the Institute of Medical Science, The University of Tokyo (approval no. 2020–5-0420). During March 1–May 14, we identified all adult hospitalized patients with COVID-19 at a university hospital in Tokyo, Japan. We confirmed diagnoses of COVID-19 by using reverse transcription PCR. Patients who had known factors that could affect pulse rate (e.g., concurrent conditions or medications) were excluded.
We obtained the highest body temperature in each day during hospitalization and the pulse rate at the time. To account for within-person correlation, we used 2-level mixed-effects linear regression (with random intercept) for analysis of factors associated with pulse rate: age, sex, time from first symptoms, systolic blood pressure, diastolic blood pressure, respiratory rate, and percutaneous oxygen saturation.
We performed variable selection by backward elimination using a p value of 0.05 by likelihood ratio test as the cutoff value. We performed statistical analysis by using Stata MP 15.1 (StataCorp, https://www.stata.com). Relative bradycardia was defined as an increase in pulse rate <18 beats/min for each 1°C increase in body temperature (1).
During the study period, 57 patients with COVID-19 were admitted to our hospital (Table); 3 patients were excluded (2 were receiving beta-blockers and 1 had a pulmonary embolism). The median age was 45.5 years (range 20–81 years), and 72.2% (39/54) of patients were male. Median time from the appearance of first symptoms to admission was 9 days (range 2–25 days). At admission, median body temperature was 37.2°C (range 36.1°C–39.2°C), pulse rate 84 beats/min (range 62–134 beats/min), and systolic blood pressure, 116 mm Hg (range 80–170 mm Hg). During admission, 13.0% (7/54) of patients had high fever (temperature >38.9°C), and all had a pulse rate <120 beats/min (range 72–114 beats/min).
We performed computed tomography and electrocardiography for all patients: no patients were given a diagnosis of cardiac disease. Computed tomography showed pneumonia for 49 (90.7%) patients, and 11 (20.4%) patients required oxygen therapy without intubation. A total of 24 patients received COVID-19–specific treatment (favipiravir, n = 15; hydroxychloroquine, n = 10; both drugs, n = 1); no patients received vasopressors, or corticosteroids for COVID-19. All patients improved and were discharged.
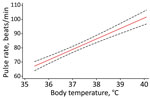
Body temperature, respiratory rate, systolic blood pressure, and time after the first symptoms (in days) were associated with pulse rate by univariable analysis (Appendix Table). However, only body temperature was independently associated with pulse rate by multivariable analysis. The predicted change in pulse rate was 7.37 (95% CI 5.92–8.82) beats/min for each 1°C increase in body temperature (Figure).
Relative bradycardia is a characteristic physical finding in some intracellular bacterial infections, viral infections, and noninfectious diseases (4). Our data showed that a predicted change in pulse rate was <18 beats/min for each 1°C increase in patients with COVID-19. Furthermore, all patients with high fever also met another criterion of relative bradycardia (i.e., body temperature >38.9°C with pulse rate <120 beats/min) (1).
Although the mechanism of relative bradycardia is not known, a hypothesis is that increased levels of inflammatory cytokines, such as interleukin-6, which was reported for patients with COVID-19, can increase vagal tone and decrease heart rate variability (4–6). Another hypothesis is that the toxic effect on the nervous system caused by SARS-CoV-2 (7) disturbs autonomic control of heart rate. Angiotensin-converting enzyme 2, which is the receptor for SARS-CoV-2, is known to be expressed on cardiac cells (8). Therefore, relative bradycardia might reflect a characteristic inflammatory response to COVID-19, directly or indirectly affecting cardiovascular system.
There are several limitations in our study. First, 34 patients received antipyretic medicines during their hospitalization (acetaminophen, n = 33; loxoprofen, n = 1), and 1 patient received prednisolone (5 mg/day) for myasthenia gravis. Because fever was underestimated for patients who received these medications, relative bradycardia might be a more common clinical sign. In our cohort, body temperature decreased over time. Although there was a relationship between pulse rate and time after first symptom in a univariable model, this finding was probably confounded by body temperature and thus not significant when adjusted. Second, our data did not include patients who were intubated. Additional research on patients with severe respiratory dysfunction is needed.
In summary, relative bradycardia was a characteristic clinical finding in patients who had mild-to-moderate COVID-19 in Japan. This clinical sign could help clinicians diagnose COVID-19.
Dr. Ikeuchi is a graduate student at the Institute of Medical Science, University of Tokyo, Tokyo, Japan. His primary research interest is HIV.
References
- Cunha BA. The diagnostic significance of relative bradycardia in infectious disease. Clin Microbiol Infect. 2000;6:633–4. DOIPubMedGoogle Scholar
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al.; China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–20. DOIPubMedGoogle Scholar
- Lian J, Jin X, Hao S, Cai H, Zhang S, Zheng L, et al. Analysis of epidemiological and clinical features in older patients with corona virus disease 2019 (COVID-19) out of Wuhan. Clin Infect Dis. 2020;Mar 25:ciaa242.
- Ye F, Hatahet M, Youniss MA, Toklu HZ, Mazza JJ, Yale S. The clinical significance of relative bradycardia. WMJ. 2018;117:73–8.PubMedGoogle Scholar
- Ye F, Winchester D, Stalvey C, Jansen M, Lee A, Khuddus M, et al. Proposed mechanisms of relative bradycardia. Med Hypotheses. 2018;119:63–7. DOIPubMedGoogle Scholar
- Hajiasgharzadeh K, Mirnajafi-Zadeh J, Mani AR. Interleukin-6 impairs chronotropic responsiveness to cholinergic stimulation and decreases heart rate variability in mice. Eur J Pharmacol. 2011;673:70–7. DOIPubMedGoogle Scholar
- Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–90. DOIPubMedGoogle Scholar
- Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–4. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: July 01, 2020
Table of Contents – Volume 26, Number 10—October 2020
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Eisuke Adachi, Department of Infectious Diseases and Applied Immunology, Institute of Medical Science, The University of Tokyo, 4-6-1 Shirokanedai, Minato-ku, Tokyo, Japan
Top