Volume 27, Number 5—May 2021
Research Letter
Intersecting Paths of Emerging and Reemerging Infectious Diseases
Cite This Article
Citation for Media
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) shares common clinicopathologic features with other severe pulmonary illnesses. Hantavirus pulmonary syndrome was diagnosed in 2 patients in Arizona, USA, suspected of dying from infection with SARS-CoV-2. Differential diagnoses and possible co-infections should be considered for cases of respiratory distress during the SARS-CoV-2 pandemic.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease (COVID-19), emerged in Wuhan, China, during December 2019 and spread rapidly to other parts of China and the world (1). However, the clinical and pathologic features of COVID-19 are also found for other respiratory disease, such as hantavirus pulmonary syndrome (HPS). In 1993, a hantavirus (Sin Nombre virus) and its rodent reservoir (Peromyscus maniculatus deer mouse) were identified as the causative agent and vertebrate reservoir responsible for an outbreak of severe pulmonary illness, named HPS, in the Four Corners region in the southwestern United States (2–4).
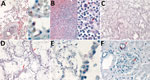
Soon after the emergence and recognition of COVID-19 in the United States in early 2020, the Infectious Diseases Pathology Branch, Division of High-Consequence Pathogens and Pathology, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention initiated diagnostic testing of fixed tissue specimens from deceased persons who had suspected or confirmed SARS-CoV-2 infection (5,6). During May 2020, Infectious Diseases Pathology Branch received tissues from an 11-year-old child (patient 1) from Arizona, who died after a brief illness culminating in severe respiratory distress. Histopathological findings included diffuse alveolar damage with rare hyaline membranes, intraalveolar edema, leukocytosis with a left shift (Figure, panel A), interstitial pneumonitis and immunoblasts in the red pulp and periarteriolar sheaths of the spleen (Figure, panel B). RNA extracted from formalin-fixed, paraffin-embedded trachea and lung tissues was positive for SARS-CoV-2 by conventional reverse transcription PCR (RT-PCR) and sequencing of positive amplicons. However, evaluation for SARS-CoV-2 by using an immunohistochemical (IHC) assay (5) showed negative results.
Subsequently, embalmed lung tissues were received from the child`s mother, a 25-year-old woman (patient 2) who died 2 days before the child after a brief illness characterized by progressive shortness of breath, cough, abdominal pain, fever, and hemoptysis. Histopathologic findings for the lungs of patient 2 resembled those identified for patient 1 (Figure, panels C, D), but there was no evidence of SARS-CoV-2 in the lung tissues by RT-PCR. Because clinicopathologic features were characteristic of HPS, we performed IHC assay for hantavirus. IHC showed typical punctate granular staining of hantaviral antigens in pulmonary and glomerular capillaries, characteristic of HPS (4) (Figure, panels E, F). IHC evaluation of lung and kidney tissues of patient 1 for hantavirus showed a similar pattern, confirming the infection in both patients (Figure, panel A, right side).
The clinicopathologic and IHC findings indicate that both patients died from HPS. Although SARS-CoV-2 RNA was detected by RT-PCR in patient 1, it was not the probable underlying cause of death. This scenario provides an essential reminder that previously recognized, nonendemic infectious diseases that clinically resemble COVID-19 continue to occur during the pandemic, in a manner similar to other clinicopathologic mimics described previously during other pandemic diseases (7).
Consideration of alternative diagnoses of diseases that precipitate acute respiratory distress syndrome and co-infections remains crucial for diagnosing and treating of critically ill patients, as well as accurately determining causes of death. For HPS, triage tools such as peripheral blood smear review and identifying 4 of 5 findings (thrombocytopenia, hemoconcentration, granulocytic left shift, absence of toxic changes, and >10% immunoblasts) can be used to diagnose the disease rapidly and presumptively in the clinical setting (8,9). Communication and partnerships of local, state, and federal public health officials and healthcare professionals, including clinicians, infectious disease specialists, pathologists, and medical examiners, are essential during these challenging times of the SARS-CoV-2 pandemic.
Ms. Wilson is a guest researcher, as part of a doctoral scholarship from the Capes-PrInt Program, in the Division of High-Consequence Pathogens and Pathology, National Center for Emerging and Zoonotic Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, GA. Her research interests include investigative and comparative pathology and pathogenesis of zoonotic and human infectious diseases.
Acknowledgment
This study was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior Brazil (Finance Code 001; doctoral scholarship for T.M.W.).
References
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al.; China Novel Coronavirus Investigating and Research Team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–33. DOIPubMedGoogle Scholar
- Duchin JS, Koster FT, Peters CJ, Simpson GL, Tempest B, Zaki SR, et al.; The Hantavirus Study Group. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. N Engl J Med. 1994;330:949–55. DOIPubMedGoogle Scholar
- Nichol ST, Spiropoulou CF, Morzunov S, Rollin PE, Ksiazek TG, Feldmann H, et al. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science. 1993;262:914–7. DOIPubMedGoogle Scholar
- Zaki SR, Greer PW, Coffield LM, Goldsmith CS, Nolte KB, Foucar K, et al. Hantavirus pulmonary syndrome: pathogenesis of an emerging infectious disease. Am J Pathol. 1995;146:552–79.
- Martines RB, Ritter JM, Matkovic E, Gary J, Bollweg BC, Bullock H, et al.; COVID-19 Pathology Working Group. Pathology and pathogenesis of SARS-CoV-2 associated with fatal coronavirus disease, United States. Emerg Infect Dis. 2020;26:2005–15. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Collection and submission of postmortem specimens from deceased persons with known or suspected COVID-19, November 2020 (Interim Guidance) [cited 2020 Oct 21]. https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-postmortem-specimens.html
- Blau DM, Denison AM, Bhatnagar J, DeLeon-Carnes M, Drew C, Paddock C, et al.; Infectious Diseases Pathology Branch Working Group. Fatal infectious diseases during pandemic (H1N1) 2009 outbreak. Emerg Infect Dis. 2011;17:2069–70. DOIPubMedGoogle Scholar
- Koster F, Foucar K, Hjelle B, Scott A, Chong YY, Larson R, et al. Rapid presumptive diagnosis of hantavirus cardiopulmonary syndrome by peripheral blood smear review. Am J Clin Pathol. 2001;116:665–72. DOIPubMedGoogle Scholar
- Dvorscak L, Czuchlewski DR. Successful triage of suspected hantavirus cardiopulmonary syndrome by peripheral blood smear review: a decade of experience in an endemic region. Am J Clin Pathol. 2014;142:196–201. DOIPubMedGoogle Scholar
Figure
Cite This ArticleOriginal Publication Date: March 11, 2021
Table of Contents – Volume 27, Number 5—May 2021
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Sherif R. Zaki, Centers for Disease Control and Prevention, 1600 Clifton Rd NE, Mailstop H18-SB, Atlanta, GA 30329-4027, USA; e-mail:szaki@cdc.gov
Top