Volume 27, Number 8—August 2021
Dispatch
Natural Human Infections with Plasmodium cynomolgi, P. inui, and 4 other Simian Malaria Parasites, Malaysia
Cite This Article
Citation for Media
Abstract
We detected the simian malaria parasites Plasmodium knowlesi, P. cynomolgi, P. inui, P. coatneyi, P. inui–like, and P. simiovale among forest fringe–living indigenous communities from various locations in Malaysia. Our findings underscore the importance of using molecular tools to identify newly emergent malaria parasites in humans.
Zoonotic malaria caused by Plasmodium knowlesi, commonly found in long-tailed macaques (Macaca fascicularis) and pig-tailed macaques (M. nemestrina), is now a major emerging disease, particularly in Malaysia (1,2). Two other simian malaria parasites, P. cynomolgi (2–4) and P. inui (2), have also been shown to have the potential of zoonotic transmission to humans through the bites of infected mosquitoes under natural and experimental conditions. The risk of acquiring zoonotic malaria is highest for persons living at the forest fringe and working or venturing into the forest because of their proximity with the monkey reservoir hosts and the mosquito vectors (5,6). With the aid of molecular methods, we aimed to investigate whether human infections with simian malaria parasites were present among indigenous communities in Malaysia whose villages are situated in the forest or at the forest fringe.
We examined 645 archived blood samples that we had collected during 2011–2014 among indigenous populations of various subtribes from 14 villages in 7 states in Malaysia (Appendix Table 1). We first screened the extracted DNA samples at Universiti Malaya (UM) for the presence of Plasmodium with the aid of genus-specific primers (rPLU1 and rPLU5; rPLU3 and rPLU4) (Appendix). Of the 645 indigenous community samples, 102 (15.8%) were positive for Plasmodium. Using species-specific nested PCR assays (Appendix), we identified these infections as monoinfections with P. knowlesi (n = 40), P. vivax (n = 21), P. cynomolgi (n = 9), P. falciparum (n = 6), P. coatneyi (n = 3), P. inui (n = 3), P. malariae (n = 2), and P. ovale curtisi (n = 1) (Table 1). In 17 samples, the species could not be identified despite repeated attempts. Our species-specific primer pairs were designed on the basis of either the asexually (A) or sexually (S) transcribed forms of Plasmodium small subunit (SSU) rRNA genes (7); the genus-specific primer pairs anneal to both asexual and sexual forms of the SSU rRNA genes, and therefore the genus-specific assay is more sensitive.
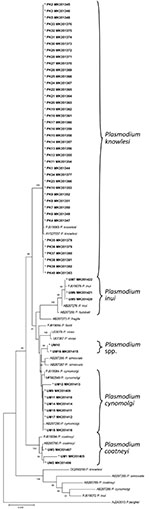
We further characterized the 55 samples that tested positive for simian malaria parasites by amplifying a longer fragment of the SSU rRNA gene (914 bp–950 bp) for direct sequencing. Phylogenetic analysis using the neighbor-joining method (Figure 1) revealed the presence of P. knowlesi (samples PK1–40), P. coatneyi (UM1–3), P. cynomolgi (UM9, UM11, UM12, UM14, UM15, UM17, UM18), and P. inui (UM5–7). Meanwhile, 2 sequences derived from samples UM10 and UM16 were found to be closely related to P. simiovale.
We then reextracted DNA from 15 blood samples that were positive for P. coatneyi, P. cynomolgi, and P. inui and sent these samples (blinded) together with 5 Plasmodium-negative samples to Universiti Malaysia Sarawak (UNIMAS) to confirm their identities by PCR and sequencing of part of the cytochrome c oxidase subunit 1 (COX1) gene. At UNIMAS, using nested PCR assays based on SSU rRNA genes, we found 1 single and 9 double species infections. We could not identify the species of Plasmodium for sample UM6, 4 of the Plasmodium-positive samples from UM were Plasmodium negative, and all 5 Plasmodium-negative samples from UM (UM4, 8, 13, 19, 20) tested negative (Table 2). Furthermore, because both laboratories at UM and UNIMAS had previously extracted DNA from macaque blood to examine for simian malaria parasites, we tested the samples for macaque DNA to rule out the possibility that the simian malaria parasites detected were the result of contamination with macaque blood. We obtained negative results using nested PCR for detection of macaque DNA for the 20 DNA samples when they were first received at UNIMAS and also when we repeated testing after completing the sequencing of COX1 genes, indicating that these samples were not contaminated with macaque blood upon receipt or during subsequent experiments at UNIMAS.
We then subjected the PCR-positive samples (UM6–7, UM9–12, UM14–18) to amplification and sequencing of partial COX1 genes. Neighbor-joining (Figure 2) phylogenetic inference of these sequences, together with available referral sequences from GenBank, indicated that 32 haplotypes from samples UM9–12 and UM14–18 were genetically indistinguishable from P. cynomolgi. Our phylogenetic analyses also demonstrated that sample UM7 had a single infection with P. inui–like parasites, whereas UM6 had a double infection with P. simiovale and P. inui–like parasites and UM16 had a triple infection with P. cynomolgi, P. simiovale, and P. inui–like parasites.
We generated phylogenetic trees of similar topology by the maximum-likelihood method for the SSU rRNA genes (Appendix Figure 1) and by the Bayesian maximum clade credibility method for the COX1 genes (Appendix Figure 2). There were discrepancies between the nested PCR assay results and the sequencing results between our 2 laboratories; mixed species of Plasmodium were identified only at UNIMAS. A possible explanation is that the DNA samples analyzed at UNIMAS were newly extracted and were different from the ones used in the experiments at UM. There might also be a compromise of the sensitivity in detecting the species with lower parasitemia in mixed infections as a result of competition for nest 1 primers by the species with higher parasite loads. Furthermore, for sequencing of the SSU rRNA genes at UM, primers that were specific for the species identified by nested PCR assays were used, whereas for the COX1 genes, both P. cynomolgi–specific primers and primers that could amplify other species of Plasmodium were used. Therefore, additional species of Plasmodium were identified at UNIMAS in these samples, such as P. simiovale and P. inui–like, for which no species-specific PCR primers exist.
The 40 P. knowlesi infections we detected originated from 6 states in Malaysia, thereby confirming the widespread distribution of human P. knowlesi malaria cases in Malaysia (1). We detected P. cynomolgi infections among indigenous communities in 4 states in Malaysia. Taken together with previous reports of naturally acquired P. cynomolgi infections in humans in the states of Terengganu, Sabah, and Sarawak (3,8,9), our findings indicate that human infections caused by P. cynomolgi are also widely distributed in Malaysia.
Our study highlights the occurrence of naturally acquired human infections with P. inui, P. inui–like, P. coatneyi, and P. simiovale. Natural human P. inui infections have not been described (10), although the parasite is experimentally transmissible to humans (2). For P. coatneyi, attempts to infect humans with blood from an infected rhesus monkey and through infected mosquitoes were unsuccessful (2). P. simiovale is a lesser-studied simian malaria parasite that was previously described only in toque macaques (Macaca sinica) of Sri Lanka (2) until it was recently identified, together with P. inui–like parasites, in long-tailed macaques from Sarawak in Malaysian Borneo (11). All these simian malaria parasites would have been diagnosed by microscopy as human malaria parasites because they share morphological similarities with human malaria parasites. The early blood stages of P. knowlesi resemble those of P. falciparum, and the other forms are similar to P. malariae (2,6). P. cynomolgi is morphologically similar to P. vivax (2), and both P. inui and P. inui–like parasites are morphologically identical to P. malariae (2,11), whereas P. coatneyi bears morphologic similarities to P. falciparum and P. simiovale bears morphologic similarities to P. ovale (2,12). Besides misdiagnosis of simian malaria parasites as human malaria parasites, there are other limitations of microscopy for diagnosis of malaria; thus, utilization of molecular tools is paramount in generating accurate epidemiology data (6). It is envisaged that screening with molecular tools of other communities living at the forest fringes will demonstrate the widespread distribution of zoonotic malaria and uncover more newly emergent malaria parasites.
Dr. Yap is a postdoctoral researcher at the Department of Parasitology, Faculty of Medicine, Universiti Malaya, Kuala Lumpur, Malaysia. Her primary research interests include epidemiology and molecular characterization of zoonotic malaria species.
Acknowledgments
We thank the staff from the Department of Orang Asli Development, Ministry of Rural Development, Malaysia, for assisting in the collection of blood samples from communities in rural areas.
The research was supported by funding from Universiti Malaya Student Grant (no. PG056-2013A to N.J.Y.), UM/MoHE High Impact Research Grant (no. H-20001-00-E000061 to Y.A.L.), and UNIMAS Special Top Down Grant (no. F05/TDG/1734/2018 to B.S.).
References
- Jeyaprakasam NK, Liew JWK, Low VL, Wan-Sulaiman WY, Vythilingam I. Plasmodium knowlesi infecting humans in Southeast Asia: What’s next? PLoS Negl Trop Dis. 2020;14:
e0008900 . DOIPubMedGoogle Scholar - Coatney GR, Collins WE, Warren M, Contacos PG. The primate malarias. Washington (DC): US National Institute of Allergy and Infectious Diseases; 1971
- Ta TH, Hisam S, Lanza M, Jiram AI, Ismail N, Rubio JM. First case of a naturally acquired human infection with Plasmodium cynomolgi. Malar J. 2014;13:68. DOIPubMedGoogle Scholar
- Imwong M, Madmanee W, Suwannasin K, Kunasol C, Peto TJ, Tripura R, et al. Asymptomatic natural human infections with the simian malaria parasites Plasmodium cynomolgi and Plasmodium knowlesi. J Infect Dis. 2019;219:695–702.
- Anstey NM, Grigg MJ. Zoonotic malaria: the better you look, the more you find. J Infect Dis. 2019;219:679–81. DOIPubMedGoogle Scholar
- Singh B, Daneshvar C. Human infections and detection of Plasmodium knowlesi. Clin Microbiol Rev. 2013;26:165–84. DOIPubMedGoogle Scholar
- Li J, Gutell RR, Damberger SH, Wirtz RA, Kissinger JC, Rogers MJ, et al. Regulation and trafficking of three distinct 18 S ribosomal RNAs during development of the malaria parasite. J Mol Biol. 1997;269:203–13. </jrn>
- Grignard L, Shah S, Chua TH, William T, Drakeley CJ, Fornace KM. Natural human infections with Plasmodium cynomolgi and other malaria species in an elimination setting in Sabah, Malaysia. J Infect Dis. 2019;220:1946–9. DOIPubMedGoogle Scholar
- Raja TN, Hu TH, Kadir KA, Mohamad DSA, Divis PCS, Wong LL, et al. Naturally acquired human Plasmodium cynomolgi and P. knowlesi infections, Malaysian Borneo. Emerg Infect Dis. 2020;26:1801–9.
- Siner A, Liew ST, Kadir KA, Mohamad DSA, Thomas FK, Zulkarnaen M, et al. Absence of Plasmodium inui and Plasmodium cynomolgi, but detection of Plasmodium knowlesi and Plasmodium vivax infections in asymptomatic humans in the Betong division of Sarawak, Malaysian Borneo. Malar J. 2017;16:417.
- Nada Raja T, Hu TH, Zainudin R, Lee KS, Perkins SL, Singh B. Malaria parasites of long-tailed macaques in Sarawak, Malaysian Borneo: a novel species and demographic and evolutionary histories. BMC Evol Biol. 2018;18:49. DOIPubMedGoogle Scholar
- Dissanaike AS. Simian malaria parasites of Ceylon. Bull World Health Organ. 1965;32:593–7.PubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: June 30, 2021
1These authors contributed equally to this article.
Table of Contents – Volume 27, Number 8—August 2021
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Addresses for correspondence: Yvonne Ai-Lian Lim, Department of Parasitology, Faculty of Medicine, Universiti Malaya, Kuala Lumpur, Malaysia
Top