Volume 28, Number 1—January 2022
Dispatch
SARS-CoV-2 RNA Shedding in Semen and Oligozoospermia of Patient with Severe Coronavirus Disease 11 Weeks after Infection
Abstract
We report severe acute respiratory syndrome coronavirus 2 in semen by using quantitative reverse transcription PCR during the late convalescent phase. Virus was associated with adequate humoral and cell-mediated responses, suggesting possible seeding of the immune-privileged testes. We provide longitudinal semen quality data for 6 other men, including 3 who had oligozoospermia.
As of July 2021, >180 million persons worldwide were infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and remain in the convalescent phase (1). Long-term implications for male fertility and potential sexual transmission remain uncertain. However, other emerging pathogens, such as Ebola and Zika viruses, have been shown to undergo sexual transmission (2,3).
Angiotensin-converting enzyme-2 receptors, abundant in the testis (4), are binding sites for SARS-CoV-2, and autopsy reports have demonstrated viral invasion of the testis (5). However, detection of SARS-CoV-2 RNA in semen was not reported from 6 cohort studies that included 213 men (6), although detection has been reported in 2 studies during the acute phase (7,8) and in 1 study during early convalescence (9). We present findings of semen analysis from a prospective coronavirus disease (COVID-19) cohort study.
A 34-year-old man, who had a history of childhood asthma, was hospitalized for severe coronavirus disease (COVID-19) pneumonia during March 2020. At admission, he had symptoms for 1 week and was positive for SARS-CoV-2 RNA by nasopharyngeal swab specimen reverse transcription PCR (RT-PCR). Chest radiograph showed bilateral interstitial opacities. He was given supplementary oxygen by nasal cannula and completed a 5-day course of hydroxychloroquine but was also given mechanical ventilation on day 10 for respiratory decompensation. His hospital course was complicated by renal failure requiring continuous venovenous hemofiltration. He was eventually extubated on illness day 15 but remained admitted until day 27, when he no longer required supplemental oxygen. Despite his respiratory recovery, he required outpatient dialysis until day 51. At the time of study enrollment (day 72), he had returned to his previous state of health.
Participants were recruited for this prospective cohort study from inpatient and outpatient settings in New York, New York, beginning in March 2020. The study was approved by the institutional review board at Columbia University Irving Medical Center and is registered at Clinicaltrials.gov (NCT04448145). Eligible participants had laboratory confirmation of COVID-19 based on SARS-CoV-2 RT-PCR or serologic testing. Participants completed surveys describing their demographics, underlying conditions, and COVID-19 clinical course. Clinical samples collected at each visit included plasma, peripheral blood mononuclear cells, nasopharyngeal swab specimens, saliva samples, stool/rectal swab specimens, and semen.
We collected and assessed semen per World Health Organization guidelines (10). We instructed participants to clean their hands without spermotoxic lubricants before providing a sample into a sterile container. Samples were frozen after collection. Sperm count was reported in millions per milliliter, and sperm motility was reported as a percentage, according to World Health Organization guidelines.
Of the 107 patients enrolled in the cohort study, 7 provided semen specimens (Table 1; Appendix). The mean age of participants was 38.7 (range 32–56) years. Two of the 7 who provided semen specimens were Hispanic, 2 Black, and 3 Caucasian; 2 had a previous diagnoses of HIV infection, and 2 had body mass index >30 kg/m2. One participant had had a successful vasectomy and was included in the study to evaluate viral carriage in nontestes accessory organs, such as the prostate gland.
A total of 17 semen specimens were collected from 7 participants (Table 1) and underwent quantitative RT-PCR (qRT-PCR) testing and semen analysis. We assessed cycle threshold (Ct) values by using the ZymoBIOMICS DNA/RNA Extraction Kit (Zymoresearch, https://www.zymoresearch.com), the Taqman 4× One-Step Master Mix (Zymoresearch), and SARS-CoV-2 IDT primer/probe sets (Integrated DNA Technologies, https://www.idtdna.com). One sample obtained from participant 1 on day 81 from symptom onset was qRT-PCR positive and had a Ct value of 34.79. Virus isolation was unsuccessful (Appendix). Subsequent semen samples from the participant at days 101 and 169 were negative, as were his saliva, stool, and plasma samples (Table 2). All semen samples from the other 6 men were negative by qRT-PCR.
Participant 1 had severe oligozoospermia and a sperm concentration of <1 million/mL on day 81, followed by a gradual recovery to 16 million/mL on day 101 and 72 million/mL on day 170. All his samples showed sperm motility of 0%, although samples were previously frozen. In addition, 2 other participants had severe oligozoospermia (<5 million/mL) and 1 had mild oligozoospermia (<15 million/mL). Follow-up samples were available for 5 participants. Early sperm count recovery was observed in 3 participants, but 2 participants had a decrease in sperm count later in convalescence, and 1 participant had a count of 16 million/mL at 11 months (Table 1).
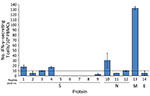
We assessed humoral and cell-mediated immune responses to evaluate level of immunity against SARS-CoV-2. We used immunoassays (11) to quantify IgM, IgG, and IgA binding (half of maximum effect) values against spike trimer and nucleocapsid protein (Figure 1). We performed antibody neutralization assays to measure the neutralization half-maximal inhibitory concentration (Figure 1; Appendix). Half of the maximum effect binding antibody responses and neutralization half-maximal inhibitory concentration against SARS-CoV-2 trimer were modest at days 72 and 78 (Table 2). We used a human interferon-γ ELISpot assay to determine the T-cell response against spike trimer, nucleocapsid, matrix, and envelope proteins at day 81 from symptom onset, which showed strong reactivity against matrix (Figure 2).
We report detection of SARS-CoV-2 RNA by qRT-PCR in semen and severe oligozoospermia in 1 patient 81 days after onset of severe COVID-19. Compared with reports that showed positive RT-PCR findings (7–9), we provide more granularity regarding clinical course, longitudinal assessment of sperm count, and host immune response.
Detection of SARS-CoV-2 RNA during the late convalescent phase might be attributed to COVID-19 severity, requiring mechanical ventilation and renal replacement therapy for the participant. This feature might have resulted in an enhanced systemic viremic state with subsequent seeding of accessory organs or the testes, an immune-privileged site, in the setting of generalized inflammation, and disruption of the blood–testis barrier (12). This possibility is supported by the participant having adequate humoral and cell-mediated immune responses and associated viral clearance of stool and saliva specimens surrounding the time when SARS-CoV-2 RNA was detected in semen.
In addition, 4 other study participants had oligozoospermia. Sperm count recovery was observed in 2 participants, but the other 2 did not provide longitudinal samples. Numerous reports have suggested a detrimental effect on semen quality after COVID-19 (9,13,14), hypothesized to occur secondary to viral illness and fever causing spermatogenic dysfunction (15). Given this transient insult, it is not unexpected that some of these men showed recovery.
One limitation of our study is that the initial semen sample was collected late in the convalescent phase, and the high Ct value probably indicates detection of inactive virus without risk for sexual transmission. However, if an acute-phase or early convalescent-phase specimen were collected, the Ct value might have been lower. Likewise, semen samples from the other 6 men were also limited to the late convalescence phase and mild acute COVID-19 illnesses, except for 1 participant who required mechanical ventilation, although his status was postvasectomy.
Given the small sample size, we cannot determine the contribution of other known etiologies of oligozoospermia, including obesity and oxygen therapy during hospitalization. In addition, we lacked preinfection semen analysis for comparison, and sperm motility would have been more accurately assessed if performed before freezing. Last, because of inherent difficulty in recruiting for serial semen collection, semen was only collected from a small proportion of participants enrolled in the cohort study. These findings are not generalizable to all male COVID-19 survivors and warrant further research.
In conclusion, SARS-CoV-2 RNA in semen appears to be an extremely rare event, but oligozoospermia has been reported more frequently. Risk factors for viral persistence in the male reproductive tract, longitudinal effects on semen quality, and viral transmission remain to be elucidated, but because of the large number of men in the convalescent phase worldwide, potential effects on reproductive health is not negligible.
Dr. Purpura is an instructor in medicine in the Department of Medicine, Division of Infectious Disease, Columbia University Irving Medical Center, New York, NY. His primary research interests include postinfectious sequelae and emerging pathogens.
Acknowledgments
We thank all persons who participated in this study and Nahid Punjani for providing technical guidance in the preparation of this manuscript.
L.J.P. was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (award T32AI114398).
References
- Medicine JHU. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE), 2021 [cited 2021 Apr 15]. https://coronavirus.jhu.edu/map.html
- Kurscheidt FA, Mesquita CSS, Damke GMZF, Damke E, Carvalho ARBA, Suehiro TT, et al. Persistence and clinical relevance of Zika virus in the male genital tract. Nat Rev Urol. 2019;16:211–30. DOIPubMedGoogle Scholar
- Sneller MC, Reilly C, Badio M, Bishop RJ, Eghrari AO, Moses SJ, et al.; PREVAIL III Study Group. A longitudinal study of Ebola sequelae in Liberia. N Engl J Med. 2019;380:924–34. DOIPubMedGoogle Scholar
- Shen Q, Xiao X, Aierken A, Yue W, Wu X, Liao M, et al. The ACE2 expression in Sertoli cells and germ cells may cause male reproductive disorder after SARS-CoV-2 infection. J Cell Mol Med. 2020;24:9472–7. DOIPubMedGoogle Scholar
- Ma X, Guan C, Chen R, Wang Y, Feng S, Wang R, et al. Pathological and molecular examinations of postmortem testis biopsies reveal SARS-CoV-2 infection in the testis and spermatogenesis damage in COVID-19 patients. Cell Mol Immunol. 2021;18:487–9. DOIPubMedGoogle Scholar
- Hezavehei M, Shokoohian B, Nasr-Esfahani MH, Shpichka A, Timashev P, Shahverdi AH, et al. Possible male reproduction complications after coronavirus pandemic. Cell J. 2021;23:382–8.PubMedGoogle Scholar
- Li D, Jin M, Bao P, Zhao W, Zhang S. Clinical characteristics and results of semen tests among men with Coronavirus Disease 2019. JAMA Netw Open. 2020;3:
e208292 . DOIPubMedGoogle Scholar - Machado B, Barcelos Barra G, Scherzer N, Massey J, Dos Santos Luz H, Henrique Jacomo R, et al. Presence of SARS-CoV-2 RNA in semen-cohort study in the United States COVID-19 positive patients. Infect Dis Rep. 2021;13:96–101. DOIPubMedGoogle Scholar
- Gacci M, Coppi M, Baldi E, Sebastianelli A, Zaccaro C, Morselli S, et al. Semen impairment and occurrence of SARS-CoV-2 virus in semen after recovery from COVID-19. Hum Reprod. 2021;36:1520–9. DOIPubMedGoogle Scholar
- World Health Organization. WHO laboratory manual for the examination and processing of human semen, fifth ed. Geneva: The Organization; 2010.
- Wang P, Liu L, Nair MS, Yin MT, Luo Y, Wang Q, et al. SARS-CoV-2 neutralizing antibody responses are more robust in patients with severe disease. Emerg Microbes Infect. 2020;9:2091–3. DOIPubMedGoogle Scholar
- Mruk DD, Cheng CY. The mammalian lood-testis barrier: its biology and regulation. Endocr Rev. 2015;36:564–91. DOIPubMedGoogle Scholar
- Holtmann N, Edimiris P, Andree M, Doehmen C, Baston-Buest D, Adams O, et al. Assessment of SARS-CoV-2 in human semen-a cohort study. Fertil Steril. 2020;114:233–8. DOIPubMedGoogle Scholar
- Best JC, Kuchakulla M, Khodamoradi K, Lima TFN, Frech FS, Achua J, et al. Evaluation of SARS-CoV-2 in human semen and effect on total sperm number: a prospective observational study. World J Mens Health. 2021;39:489–95. DOIPubMedGoogle Scholar
- Sergerie M, Mieusset R, Croute F, Daudin M, Bujan L. High risk of temporary alteration of semen parameters after recent acute febrile illness. Fertil Steril. 2007;88:970.e1–7. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: October 13, 2021
Table of Contents – Volume 28, Number 1—January 2022
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Lawrence J. Purpura, Columbia University Irving Medical Center, 622 W 168th St, PH 904, New York, NY 10032, USA
Top