Volume 28, Number 10—October 2022
Dispatch
Nosocomial COVID-19 Incidence and Secondary Attack Rates among Patients of Tertiary Care Center, Zurich, Switzerland
Abstract
Of 1,118 patients with COVID-19 at a university hospital in Switzerland during October 2020–June 2021, we found 83 (7.4%) had probable or definite healthcare-associated COVID-19. After in-hospital exposure, we estimated secondary attack rate at 23.3%. Transmission was associated with longer contact times and with lower cycle threshold values among index patients.
Since the beginning of the COVID-19 pandemic, hospitals have introduced infection prevention and control (IPC) measures to protect inpatients from SARS-CoV-2. Despite these precautions, healthcare-associated COVID-19 has affected a notable proportion of hospitalized patients (1–3). During the second and third waves of COVID-19 in Switzerland, an increasing number of patients with presymptomatic or asymptomatic COVID-19 exposed hospital roommates to SARS-CoV-2. We estimated the secondary attack rate (SAR) after exposure in a hospital in Zurich and identified risk factors for SARS-CoV-2 transmission.
University Hospital Zurich, a 900-bed tertiary care center, implemented intensified standard precaution measures during the COVID-19 pandemic (Appendix). We included in our analysis all patients with COVID-19 admitted during the second and third COVID-19 waves, weeks 40 of 2020 through 25 of 2021; a small percentage were also included in a study investigating transmission to healthcare workers (4). We stratified COVID-19 sources as community-associated, healthcare-associated (definite and probable), or indeterminate according to European Centre for Disease Prevention and Control criteria (5). We defined index patients as those who, during the 48 hours before onset of signs and symptoms or a positive SARS-CoV-2 test, had contact with an exposed patient. We defined exposed patients as those sharing a room with an index patient for >6 hours in an intermediate care unit (IMC) or intensive care unit (ICU) (1), any time on the general ward, or when the index patient underwent an aerosol-generating procedure (2). We initiated droplet isolation precaution measures for exposed patients and tested them upon symptom onset or, beginning week 47 of 2020, systematically at 2, 5, and 10 days after exposure. We contacted discharged patients by phone and offered testing in the outpatient clinic. Patients with up-to-date vaccination or known COVID-19 during the previous 6 months were considered unexposed.
We assessed transmission pathways between patients by in-depth reviews of symptom onset and dynamics of cycle threshold (Ct) values. If sequencing results were available, evidence of transmission was defined as ≤1 single-nucleotide polymorphism (SNP). Exposed patients with >1 SNP difference from the index patient were excluded from the main analysis, but patients without sequencing data were included. For association with SARS-CoV-2 transmission, we assessed index patient age, sex, aerosol generating procedures, and Ct values from first positive PCR test, duration of contact between an index patient and exposed patient, ward type, and pandemic week.
Routine SARS-CoV-2 PCR testing was conducted in 3 laboratories and whole-genome sequencing performed according to nCoV-2019 sequencing protocol v3 (LoCost) V.3 (https://www.protocols.io/view/ncov-2019-sequencing-protocol-v3-locost-bp2l6n26rgqe/v3) (Appendix). To estimate SAR, we calculated cumulative incidence using the Kaplan-Meier estimator. We assessed risk factors for transmission in univariate and multivariable logistic regression models. We conducted sensitivity analyses on patients with >10 days clinical or laboratory follow-up (Appendix Table 1), on all patients irrespective of phylogenetic results (Appendix Table 2), and on patients with phylogenetically proven transmission (Appendix Table 3). We conducted analyses using Stata statistical software release 16 (StataCorp LLC, https://www.stata.com) and R version 4.0.2 (https://cran.r-project.org/bin/windows/base). The Zurich Cantonal Ethics Commission waived formal ethics evaluation because our analysis was part of an outbreak investigation for quality control and infection prevention (Req 2021-00560).
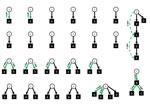
Of 1,118 patients with COVID-19, a total of 1,012 (90.5%) cases were community-associated, 40 (3.6%) probable healthcare-associated, 43 (3.8%) definite healthcare-associated, and 23 (2.1%) indeterminate (Figure 1). In total, we found 127 index patients for 303 exposed patients. Phylogenetic data supported transmission in 14/23 pairs of index–exposed patients with epidemiologic links for whom we had available data (Appendix Figure 1). In addition, we confirmed 4 transmissions indirectly by data from transmission chains (Figure 2). We excluded 5 exposed patients from the analysis because of >1 SNP difference between index and exposed patient. Among exposed patients in the analysis, 42/298 (14.1%) tested positive for SARS-CoV-2 and 179/298 (69.9%) had a follow-up time <10 days. Cumulative incidence for COVID-19 as an estimator of the SAR was 23.3% (95% CI 16%–30%) (Appendix Figure 2). Clusters were small, with only 2 multigeneration transmissions (Figure 2). We found links to identified index patients for 26 (65%) patients with probable and 15 (34.9%) patients with definite healthcare-associated COVID-19.
We performed univariable and multivariable analyses to explore factors associated with transmission of SARS-CoV-2 from patients to roommates (Table). We found similar results for 2 of the 3 sensitivity analyses (Appendix Tables 2, 3). However, in analysis of patients with a follow-up ≥10 days (Appendix Table 1), exposure on IMC/ICUs and higher number of weeks into the COVID-19 pandemic were associated with a lower risk for transmission, likely because of greater physical distance between immobile patients on the IMC/ICU and increased IPC standards.
In a mostly unvaccinated population in which most infections were caused by pre-Alpha variant SARS-CoV-2 (6), we found that 7.4% of all COVID-19 patients had probable or definite healthcare-associated COVID-19. This finding is comparable to that from the second wave in Brazil (8.6%) (7) but lower than that from the first wave in the United Kingdom (9%–15%) (2,3). We were able to link only half of the healthcare-associated cases in our hospital to an identified index patient. Despite all the IPC measures in place, high population incidence probably contributed to an increased risk for healthcare-associated transmissions from other patients but also from visitors and HCWs.
We identified ≈11% of all COVID-19 patients as index patients and estimated a 23% SAR in exposed patients. From the 23 epidemiologically linked pairs with available phylogenetic data, transmission was endorsed in only 18, suggesting that the overall index-to-contact patient transmission rate may have been overestimated. SARs among hospital roommates in a study from a tertiary care center in Iowa, USA, was 21.6% (8); from a tertiary care center in New York, New York, USA, 18.9% (9); and from a hospital in Boston, Massachusetts, USA, 39% (10). These SAR numbers are comparable to those in households (11), implying either that distancing and masking are of limited effectiveness for preventing transmission while sharing accommodations (supporting an aerosol transmission pathway between patients) (13) or that adherence to distancing and masking were low. Unsurprisingly, as also demonstrated elsewhere (8,10), the 2 parameters most strongly associated with SARS-CoV-2 were longer contact time between index and exposed patients and low Ct values (i.e., high viral loads) among index patients; Ct values <21 were shown to be associated with transmission (10).
Among limitations in our study, phylogenetic results were available for only half of the patients, laboratory follow-up with inpatients was only 10 days, and discharged patients were often not available for further follow-up. We also limited contact time on IMC/ICUs to >6 hours, which might have excluded relevant contacts, and we might have missed superinfection. Finally, we were unable to model potential drivers for transmission, such as patient nonadherence to IPC-measures, distance between index and exposed patients, or respiratory signs or symptoms of index patients.
High viral loads among index patients and prolonged contact time in shared hospital rooms play critical roles in healthcare-associated SARS-CoV2-transmission. Although based on data from a time when pre-Alpha and Alpha variants circulated in a nonvaccinated population, our findings might be relevant in the context of more recently emerged and future variants of concern (13,14) and waning immunity (15). The findings in our study and other studies of substantial SARs in hospitals support early adoption strategies to prevent healthcare-associated transmission during times of high population COVID-19 incidence. Those strategies include identifying contagious patients early (e.g., by performing systematic and repetitive SARS-CoV-2 testing), improving mask-wearing adherence in patients, and frequently replacing air in shared patient rooms.
Dr. Wolfensberger is an infectious disease physician and hospital epidemiologist at the University Hospital Zürich. Her primary research interests include outbreak investigations and hospital-acquired pneumonia.
Acknowledgments
P.W.S. is supported by the Filling the Gap academic career program of the medical faculty of the University of Zürich. This study was supported by the clinical research priority Comprehensive Genomic Pathogen Detection Program of the University of Zurich.
A.W., S.K.R., and W.Z. designed the study. V.K., M.Za., I.N., P.W.S., M.V., V.S., T.S., and D.S. acquired the data, and A.W., M. Ze., and P.W.S. performed statistical analysis. V.K., M.Za., E.P., and S.S. performed laboratory analyses. A.W., V.K., M.Za., M. Ze., and W.Z. analyzed and interpreted the data. A.W. and V.K. drafted the manuscript, and M.Za., M. Ze., I.N., P.W.S., M.V., V.S., T.S., S.S., E.P., D.S., M.H., S.K.R., and W.Z. provided critical review of the manuscript for intellectual content.
References
- Luong-Nguyen M, Hermand H, Abdalla S, Cabrit N, Hobeika C, Brouquet A, et al. Nosocomial infection with SARS-Cov-2 within departments of digestive surgery [in French]. J Chir Visc Surg. 2020;157:S13–9. DOIGoogle Scholar
- Rickman HM, Rampling T, Shaw K, Martinez-Garcia G, Hail L, Coen P, et al. Nosocomial transmission of coronavirus disease 2019: a retrospective study of 66 hospital-acquired cases in a London teaching hospital. Clin Infect Dis. 2021;72:690–3. DOIPubMedGoogle Scholar
- Taylor J, Rangaiah J, Narasimhan S, Clark J, Alexander Z, Manuel R, et al. Nosocomial COVID-19: experience from a large acute NHS Trust in South-West London. J Hosp Infect. 2020;106:621–5. DOIPubMedGoogle Scholar
- Zeeb M, Weissberg D, Rampini SR, Müller R, Scheier T, Zingg W, et al. Identifying risk contacts for SARS-CoV-2 transmission to healthcare-workers during a COVID-19 ward outbreak. Emerg Infect Dis. 2022. In press.
- European Centre for Disease Prevention and Control. Surveillance definitions for COVID-19: source of infection: healthcare (nosocomial) vs community transmission [cited 2022 Aug 16] https://www.ecdc.europa.eu/en/covid-19/surveillance/surveillance-definitions
- Federal Office of Public Health Switzerland. COVID-19 Switzerland information on the current situation [cited 2022 Aug 16]. https://www.covid19.admin.ch/en
- Tauffer J, Konstantyner TCRO, de Almeida MCS, Ferreira DB, Antonelli TS, Fram DS, et al. Impact of In-Hospital infection with SARS-CoV-2 among Inpatients at a university hospital. Am J Infect Control. 2021;49:1464–8. DOIPubMedGoogle Scholar
- Trannel AM, Kobayashi T, Dains A, Abosi OJ, Jenn KE, Meacham H, et al. Coronavirus disease 2019 (COVID-19) incidence after exposures in shared patient rooms in a tertiary-care center in Iowa, July 2020-May 2021. Infect Control Hosp Epidemiol. 2021;•••:1–4. DOIPubMedGoogle Scholar
- Chow K, Aslam A, McClure T, Singh J, Burns J, McMillen T, et al. Risk of healthcare-associated transmission of SARS-CoV-2 in hospitalized cancer patients. Clin Infect Dis. 2022;74:1579–85. DOIPubMedGoogle Scholar
- Karan A, Klompas M, Tucker R, Baker M, Vaidya V, Rhee C. The risk of SARS-CoV-2 transmission from patients with undiagnosed Covid-19 to roommates in a large academic medical center. Clin Infect Dis. 2022;74:1097–100. DOIPubMedGoogle Scholar
- Fung HF, Martinez L, Alarid-Escudero F, Salomon JA, Studdert DM, Andrews JR, et al.; Stanford-CIDE Coronavirus Simulation Model (SC-COSMO) Modeling Group. The household secondary attack rate of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): a rapid review. Clin Infect Dis. 2021;73(Suppl 2):S138–45. DOIPubMedGoogle Scholar
- Greenhalgh T, Jimenez JL, Prather KA, Tufekci Z, Fisman D, Schooley R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. [Erratum in Lancet. 2021;397:1808.]. Lancet. 2021;397:1603–5. DOIPubMedGoogle Scholar
- Ai J, Zhang H, Zhang Y, Lin K, Zhang Y, Wu J, et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg Microbes Infect. 2022;11:337–43. DOIPubMedGoogle Scholar
- Zhang L, Li Q, Liang Z, Li T, Liu S, Cui Q, et al. The significant immune escape of pseudotyped SARS-CoV-2 variant Omicron. Emerg Microbes Infect. 2022;11:1–5. DOIPubMedGoogle Scholar
- Poukka E, Baum U, Palmu AA, Lehtonen TO, Salo H, Nohynek H, et al. Cohort study of Covid-19 vaccine effectiveness among healthcare workers in Finland, December 2020 - October 2021. Vaccine. 2022;40:701–5. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: September 01, 2022
Table of Contents – Volume 28, Number 10—October 2022
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Aline Wolfensberger, Division of Infectious Diseases and Hospital Epidemiology, University Hospital Zürich, University of Zürich, Rämistrasse 100, CH-8091 Zurich, Switzerland
Top