Volume 28, Number 4—April 2022
Research
Fatal Human Alphaherpesvirus 1 Infection in Free-Ranging Black-Tufted Marmosets in Anthropized Environments, Brazil, 2012–2019
Cite This Article
Citation for Media
Abstract
Human alphaherpesvirus 1 (HuAHV1) causes fatal neurologic infections in captive New World primates. To determine risks for interspecies transmission, we examined data for 13 free-ranging, black-tufted marmosets (Callithrix penicillata) that died of HuAHV1 infection and had been in close contact with humans in anthropized areas in Brazil during 2012–2019. We evaluated pathologic changes in the marmosets, localized virus and antigen, and assessed epidemiologic features. The main clinical findings were neurologic signs, necrotizing meningoencephalitis, and ulcerative glossitis; 1 animal had necrotizing hepatitis. Transmission electron microscopy revealed intranuclear herpetic inclusions, and immunostaining revealed HuAHV1 and herpesvirus particles in neurons, glial cells, tongue mucosal epithelium, and hepatocytes. PCR confirmed HuAHV1 infection. These findings illustrate how disruption of the One Health equilibrium in anthropized environments poses risks for interspecies virus transmission with potential spillover not only from animals to humans but also from humans to free-ranging nonhuman primates or other animals.
The coronavirus disease (COVID-19) pandemic has brought the One Health concept to the forefront of global health. From an infectious disease standpoint, the focus of One Health is often on how human activity expanding and encroaching on wildlife habitats may adversely affect humans through spillover of pathogens from wildlife reservoirs. However, the opposite—transmission of pathogens from humans to wildlife—is also possible in these situations. In Brazil and other countries, destruction and alterations of natural habitats and deforestation driven by human activities such as agricultural and urban expansion force some nonhuman primate (NHP) populations to live in anthropized areas, intensifying interactions between humans and NHP species and increasing the risk for interspecies transmission of agents of infectious diseases (1).
The black-tufted marmoset (Callithrix penicillata) is one example of an NHP now well-adapted to human-altered environments. Marmosets are naturally found in the Brazilian Savanna and Caatinga Biome and are commonly commensal in urban and periurban areas (2); close human–marmoset interactions (i.e., feeding) are common. Because these settings are suitable for interspecies transmission of pathogens, infectious disease surveillance of NHPs provides an invaluable opportunity to detect emerging and reemerging zoonotic and anthroponotic diseases as well as predict pathogen spillover events.
Alphaherpesviruses usually cause asymptomatic or mild infections in their natural hosts but are often associated with severe illness after cross-transmission to new species (3). Human alphaherpesviruses (HuAHVs) consist of 2 closely related viruses, HuAHV types 1 and 2. Transmission of both HuAHV types generally requires intimate contact between actively infected and susceptible persons. Humans are natural hosts for HuAHV1, also known as herpes simplex virus 1, an alphaherpesvirus that is endemic in human populations (4). Natural fatal HuAHV1 infections are well-documented for New World primates that are in close contact with humans as pets and in captive conditions such as zoos and biomedical research centers (5–15). In contrast, HuAHV1 infections in free-ranging New World primates in anthropogenic environments are rarely reported and are limited to isolated outbreaks in urban areas (16) and in a state conservation park (17).
Captive New World primates are highly susceptible to HuAHV1 infection, and fatal disease frequently develops, characterized by mucocutaneous, facial, and oral erosions/ulcerations and meningoencephalitis (5–15). However, reports of HuAHV1 infection in free-living animals in anthropized environments are limited. We conducted a retrospective study to determine pathologic, immunohistochemical, and ultrastructural features of infection; molecular identification of the virus; and epidemiologic aspects of fatal outbreaks and isolated cases of HuAHV1 virus infection in free-ranging black-tufted marmosets in anthropized areas of the Federal District and surrounding areas of Brazil.
Case Selection
To select cases of suspected fatal herpetic infection, we reviewed 1,042 NHP necropsies performed during 2012–2019 and archived by the Veterinary Pathology Laboratory at the University of Brasilia, Federal District, and the Regional Reference Laboratory of the Brazilian Ministry of Health that performs necropsies to diagnose yellow fever as part of the National Surveillance Program of Yellow Fever Epizootics in NHP of the Brazilian Ministry of Health. For all cases, we retrieved epidemiologic information, clinical data, pathologic findings, and image data from submission forms, necropsy reports, archived images, and formalin-fixed paraffin-embedded (FFPE) tissue blocks.
Histopathologic Evaluation
Necropsy tissue samples from 13 NHPs with clinically suspected emerging infectious diseases were fixed in 10% formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. We evaluated the following samples to identify histopathologic changes consistent with suspected HuAHV infection: brain (meningitis, perivascular inflammation, neuropil inflammation with or without prominent neutrophilic component, neuronal necrosis, neuronophagy, reactive gliosis, glial nodules, and inclusion bodies within neurons and glial cells); tongue (epithelial ballooning degeneration, acantholysis, ulceration, necrosis, subepithelial inflammation, intranuclear inclusion bodies within the mucosal epithelium and syncytial cells); and liver (hepatocellular coagulative necrosis, hepatocellular eosinophilic intranuclear viral inclusion bodies, and multinucleated giant cells [syncytia]). We also recorded histopathologic changes in other organs.
Immunohistochemistry and Transmission Electron Microscopy
We performed immunohistochemistry (IHC) for HuAHV types 1 and 2 on FFPE tissues. We used a polymer-based colorimetric indirect immunoalkaline phosphatase method in deparaffinized tissue sections after rinsing the tissue sections in 1X Tris‐buffered saline with Tween 20 (1X TBS‐T; Thermo Fisher Scientific, https://www.thermofisher.com). Tissue sections were digested with 0.1 mg/mL Proteinase K (Roche, https://www.sigmaaldrich.com) in 0.6 M Tris/0.1% CaCl2 for 15 min and blocked in Background Punisher (Biocare Medical, https://biocare.net) for 10 min. Slides were incubated with a rabbit polyclonal anti-HuAHV1 and 2 antibody biological products maintained at CDC at 1:2,000 dilution, and attached antibodies were detected with Mach 4 Universal AP Polymer Kit (Biocare Medical) and Permanent Red Chromogen (Cell Marque/Millipore Sigma, https://www.cellmarque.com). We counterstained slides with Mayer hematoxylin (Polyscientific, https://www.polyrnd.com) and placed coverslips by using an aqueous mounting medium (Polysciences, Inc., https://www.polysciences.com). Appropriate positive and negative controls were run in parallel.
We also processed FFPE sections from the brain, liver, and tongue of 3 NHPs with extensive immunohistochemical evidence of HuAHV for transmission electron microscopy (TEM) analysis by using an on-slide technique. In brief, 4-μm sections of FFPE tissue affixed to glass slides that correlated with areas positively labeled for HuAHV by IHC were deparaffinized in xylene, then rehydrated and fixed in 2.5% buffered glutaraldehyde. Samples were postfixed with 1% osmium tetroxide, stained en bloc with uranyl 132 acetate, dehydrated, and embedded in Epon-Araldite resin with dibutyl phthalate (Electron Microscopy Sciences, https://www.emsdiasum.com). Epoxy resin–embedded sections on the glass slide were immersed in boiling water and removed from the slides with a razor blade; areas of interest were glued onto electron microscopy blocks. We stained ultrathin sections with uranyl acetate and lead citrate and examined them on either a Thermo Fisher/FEI Tecnai Spirit or Tecnai BioTwin electron microscope (18).
Real-Time PCRs for HuAHV1 and HuAHV2
We conducted real-time PCR for HuAHV 1 and HuAHV2 on FFPE samples with confirmed herpesvirus immunohistochemistry results. We extracted DNA from 16-μm paraffin-embedded brain or liver tissue sections from each animal by using a QIAamp UCP Pathogen Mini Kit (QIAGEN, https://www.qiagen.com) according to the manufacturer’s recommendations. Fluorescence resonance energy transfer (FRET) technology was conducted to discern HuAHV1 from HuAHV2 infections in a real-time PCR targeting the glycoprotein B, UL27 gene. The 2-probed system discriminates HuAHV1 and HuAHV2 according to analysis of the melt curves (19). We considered a sample to be HuAHV1 positive if the melt temperature was 56°C and HuAHV2 positive if 63°C. We included cases positive by FRET PCR for HuAHV1 or HuAHV2 in our study.
To amplify a 147-bp fragment of the glycoprotein B, UL27 gene from the genome of either HuAHV1 or HuAHV2, w used HuAHV1 and HuAHV2 primers (HuAHV-1/2 forward primer, 5′-TTG AAG CGG TCG GCG GCG TA- 3′; HuAHV-1/2 reverse primer, 5′-GTC CAC CTC CTC GAC GAT GC- 3′) along with the detection probe (5′-LC Red 640- GCG ACT GGC GAC TTT G- 3′-phos-cpg) and the anchor probe (5′-GGT AGC CGT AAA ACG GGG ACA TGT A- 3′-fam-cpg). PCR was performed in a 20-μL reaction volume with 0.5 μmol/L of the 50 μmol/L primer stocks, 0.2 μmol/L of the detection probe, 0.1 μmol/L of the anchor probe, and HotStarTaq DNA Polymerase and 1X QuantiTect Probe PCR Master Mix (both from QIAGEN). The PCR had the following cycling conditions: a hot start (95°C), touchdown (10 cycles of denaturation at 95°C, annealing at 62°C, and an extension at 72°C), amplification (40 cycles of denaturation at 95°C, annealing at 52°C, and an extension at 72°C), cooling at 40°C, and 1 melt cycle at 95°C. We considered a sample to be as considered positive if the melt temperature was ≈56°C for HuAHV1 and 63°C for HuAHV2. We included cases positive by FRET PCR for HuAHV1 or HuAHV2 in our study.
Real-Time PCR, Epidemiologic, and Clinical Findings
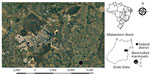
Of the 1,042 retrieved NHP cases, HuAHV fatal infection was morphologically diagnosed for 18 black-tufted marmosets (Table 1). Of these, 5 (27.8%) were from captive conditions and excluded from our study, and for the remaining 13 free-ranging marmosets, HuAHV1 was detected in all tissues tested by real-time PCR. HuAHV2 was not detected in any sample. Marmoset deaths were distributed in urban (38.5%) and peri-urban areas (61.5%) of the Federal District and Goiás State, Brazil (Figure 1). Available information indicated close contact with humans for 7 marmosets. Investigations of the probable infection site frequently showed close contact between marmosets and humans, including local residents feeding fruit to animals.
Two separate HuAHV1 outbreaks during 2012–2019 involved 9 (69%) of the 13 animals, (7 animals in outbreak 1; 2 animals in outbreak 2); the other 4 (31%) had isolated cases. In outbreak 1, an entire family group of marmosets became ill and died. Information about clinical signs was available for 12 animals. Neurologic signs were most frequently reported and included ataxia (8%), seizures (17%), muscle tremors (33%), nystagmus (8%), and anisocoria (8%). Nonspecific clinical signs were also reported, such as depression (25%), recumbency (17%), salivation (66%), and oral bleeding (8%). Fatal cases affected adults (54%) and juveniles (46%).
Pathology, Immunohistochemistry, and Electron Microscopy Findings
Gross changes retrieved from necropsy reports and photographic documentation revealed multiple cutaneous crusting erythematous erosions and ulcerations in the periocular region, lip, and tongue (Figure 2). Mandibular and cervical lymph nodes were often markedly enlarged. The most commonly affected organs, of those available for evaluation, were the brain (92%), tongue (69%), and liver (8%) (Table 2). Histopathologic findings in other organs included reactive lymph node hyperplasia (77%) and necrosis of splenic white pulp germinal centers (15%). IHC demonstrated HuAHV1 antigen in the brain of 12 (92%) marmosets, the tongue of 9 (69%), and the liver of only 1 (8%). HuAHV1 immunostaining was not detected in other representative tissue samples available, including spleen (n = 13 animals), lymph node (n = 7), heart (n = 8), kidney (n = 8), lung (n = 7), esophagus (n = 1), gastrointestinal tract (n = 7), trachea (n = 1), adrenal gland (n = 1), pancreas (n = 1), and testicle (n = 4).
Brain
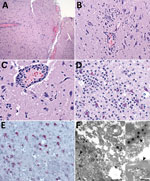
The most frequent neuropathologic finding in the HuAHV1-infected marmosets was necrotizing meningoencephalitis (Figure 3, panel A). Findings included reactive gliosis, glial nodules, neuronal necrosis, neuronophagia (Figure 3, panel B), characteristic herpetic intranuclear eosinophilic inclusion bodies within neurons and glial cells, and prominent perivascular inflammation of the gray and white matter (Figure 3, panel C). Inflammatory infiltrates varied from moderate to severe and consisted of lymphocytes, histiocytes, neutrophils, and a few plasma cells. For 3 animals, marked neutrophilic inflammation in the neuropil was detected (Figure 3, panel D). Swollen reactive endothelial cells and intravascular leukocytosis were also seen in affected areas of neuroparenchyma. Infected neurons and glial cells showed strong cytoplasmic immunostaining for HuAHV1 (Figure 3, panel E). TEM analysis of the brain revealed viral particles morphologically consistent with herpesvirus in the white and gray matter; intranuclear and cytoplasmic viral particles were also observed (Figure 3, panels F and G).
Tongue
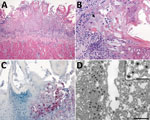
Histopathologic lesions in the tongue were multifocal, showing moderate to severe epithelial ballooning degeneration and acantholysis; multifocal to coalescing mucosal ulceration; necrosis with fibrin deposition (Figure 4, panel A); and subepithelial neutrophilic infiltrates with histiocytes, lymphocytes, and plasma cells. Epithelial cells showed rare multinucleation (syncytia formation) with nuclear molding and herpetic intranuclear eosinophilic inclusion bodies (Figure 4, panel B). Necrotic and surrounding ulcerated areas showed epithelial cells with marked cytoplasmic and nuclear viral immunostaining (Figure 4, panel C). Correlating with this viral immunostaining, TEM identified abundant herpesvirus in the nuclei and cytoplasm of epithelial cells (Figure 4, panel D).
Liver
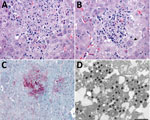
Histopathologic lesions in the liver were found in only 1 of the 13 marmosets. In this animal, necrotizing hepatitis was characterized by multifocal random coagulative necrosis, few neutrophils, and scattered hemorrhage (Figure 5, panel A). Hepatocellular eosinophilic intranuclear viral inclusion bodies and multinucleated giant cells (syncytia) were also observed (Figure 5, panels B). Necrotic foci showed strong immunolabeling for HuAHV1 antigens (Figure 5, panel C). Herpesvirus was found by TEM primarily in the cytoplasm of hepatocytes; a few nuclei also showed the presence of the virus (Figure 5, panels D).
The expansion of human activity into natural environments can disrupt the One Health equilibrium by increasing opportunities for infectious disease transmission between humans and animals. The effect of this equilibrium on human public health is often emphasized; however, disease transmission can also threaten wildlife and biodiversity in periurban settings. The 13 synanthropic free-ranging marmosets with acute, fatal HuAHV1 infections described here were from across a densely populated, anthropized environment in Brazil. Our findings corroborate those of previous reports of HuAHV1 in captive and pet marmosets and other NHPs, from clinical and pathologic standpoints, expanding the body of knowledge of HuAHV1 infection in free-ranging marmosets and highlighting the value of strategic NHP infectious disease surveillance systems.
The natural reservoir for HuAHV1 is humans, and infection of these marmosets resulted from documented or probable close interactions with humans in urban or periurban environments. HuAHV1 infection occurs by viral host epithelial invasion through direct or indirect contact with mucocutaneous lesions or with bodily secretions/excretions from asymptomatic carriers (3,6,8,20). In at least some of these marmosets, confirmed contact involved humans directly sharing food with marmosets. In the absence of direct sharing, food scraps and garbage are potential sources of HuAHV1 infection for free-ranging marmosets and other animals in anthropized environments such as the Federal District and surrounding areas. The high number of sick and dead marmosets in 1 family of marmosets in our series and in monkey families reported elsewhere suggests that animal-to-animal transmission of HuAHV1 probably also occurs (5,7–10,13–17). We found numerous viral particles within oral epithelial lesions of marmosets, suggesting high mucosal viral shedding and potential for transmission to other animals (21). Experimental HuAHV1 infection of rhesus macaques also demonstrated ocular, nasopharyngeal, oral, fecal, and urine virus shedding (22).
The clinical disease observed in these animals (neurologic signs, hypersalivation, and oral bleeding) correlated with the gross findings of glossitis and facial erythema with regional lymphadenomegaly and with histopathologic features characteristic of herpes viral meningoencephalitis and stomatitis. Similar clinical and gross findings have been reported for marmosets with fatal and nonfatal HuAHV1 infection, and necrotizing meningoencephalitis and ulcerative glossitis are the histopathologic hallmarks observed in most documented HuAHV1 outbreaks among captive and free-living NHPs (6–9,11–17,23–25).
According to histologic findings, death of these marmosets is largely attributable to the neurologic effects of infection (12,13). HuAHV1 antigen detection by IHC and viral particle detection by TEM within brain lesions confirmed viral neuroinvasiveness in these animals. Animal models have shown that primary HuAHV1 infection originates in the skin or oral mucosa, usually followed by a latent stage in sensory neurons, and finally reaching the brain through the trigeminal nerve or olfactory bulb, causing lethal encephalitis (26). Intracellular HuAHV1 replication triggers direct cytopathic effects such as programmed cell death and necrosis (27). In the 13 marmosets, brain pathology also indicated that a severe inflammatory response to viral infection played a role in disease pathogenesis (28–30). In some HuAHV1 outbreaks among marmosets, however, mortality rates may be elevated even in the absence of neuropathologic changes because marmosets are highly susceptible to infection (6). Necrotizing hepatitis with syncytia and viral inclusions, as seen in the juvenile marmoset with liver tissue available, has only rarely been reported for HuAHV1-infected NHPs (7). Similarly, a severe, disseminated disease with mucocutaneous lesions, hepatitis, and encephalitis occurs only sporadically in human neonates and severely immunocompromised patients (4) as a consequence of high viral load in hosts incapable of limiting replication at mucosal surfaces (31).
As with HuAHV1, other alphaherpesviruses typically cause mild, self-limiting disease in their natural host species but severe, generalized, and often fatal disease when cross-species transmission occurs (Table 3) (3). Among NHPs, infections of Old World monkeys compared with New World monkeys are generally more self-limiting and fatal cases more rare (23–25,28,32,33). The mechanisms underlying these variations in susceptibility are not fully known but may be associated with species differences in innate immune system function or cellular DNA repair proteins needed for the virus life cycle (34,35). Cercopithecine herpesvirus 1 (also called B virus) is the most concerning in terms of zoonotic risk, resulting in fatal human infection after transmission from macaques. Human exposure is typically reported in biomedical research settings but could also occur through interactions with macaques in anthropized natural environments (3). Analogous to B virus cross-species infection in humans, our findings reinforce the high susceptibility and severe outcomes of cross-species HuAHV1 infections in marmosets (6).
Viral latency with recrudescence is typical of alphaherpesvirus infections in the natural host, and latency can be associated with asymptomatic virus shedding (20). HuAHV1 is endemic to the human population in Brazil, and seroprevalence studies indicate high rates of infection (36). Serologic testing has also shown the potential for persistent infection in marmosets (9), implying a potential risk for spillback to humans. HuAHV1 transmission from humans to NHPs and vice versa may therefore be underrecognized; further studies are needed to determine the extent of interspecies transmission in urban and periurban settings.
In Brazil, other enzootic causes of outbreaks and acute deaths among marmosets (e.g., toxoplasmosis, rabies, and yellow fever) are included in the clinical and pathologic differential diagnosis for HuAHV infection (37–39). These other diseases are zoonotic, some of high public health concern, with possible NHP reservoir hosts. This observation exemplifies the value of public outreach and robust One Health–focused epidemiologic and pathologic surveillance programs, such as the National Surveillance Program of Yellow Fever Epizootics in NHP of the Brazilian Ministry of Health, for detecting zoonotic and anthroponotic diseases in free-ranging NHPs (40). Detection, monitoring, and predicting spillover events afforded by these programs enables development of rapid containment measures to prevent devastating new public health outbreaks and threats to wildlife populations.
Dr. Wilson is a guest researcher with the Division of High-Consequence Pathogens and Pathology, National Center for Emerging and Zoonotic Infectious Diseases, CDC, Atlanta, as part of the doctoral scholarship from Capes-PrInt program. Her research interests include investigative and comparative pathology and pathogenesis of zoonotic and human infectious diseases.
Acknowledgments
We thank Josilene Nascimento Seixas and Demi Rebeneck for sample processing and DNA extraction from FFPE tissues.
The Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brazil financed in part (finance code 001) the doctoral scholarship of T.M.W. Special thanks to The National Council for Scientific and Technological Development for financial support (grant no. 310498/2018-0 to M.B.C).
Sherif R. Zaki, senior author, died before publication of this article.
References
- Estrada A, Garber PA, Rylands AB, Roos C, Fernandez-Duque E, Di Fiore A, et al. Impending extinction crisis of the world’s primates: Why primates matter. Sci Adv. 2017;3:
e1600946 . DOIPubMedGoogle Scholar - Duarte MH, Vecci MA, Hirsch A, Young RJ. Noisy human neighbours affect where urban monkeys live. Biol Lett. 2011;7:840–2. DOIPubMedGoogle Scholar
- Tischer BK, Osterrieder N. Herpesviruses—a zoonotic threat? Vet Microbiol. 2010;140:266–70. DOIPubMedGoogle Scholar
- World Health Organization. Herpes simplex virus [cited 2021 Oct 8]. https://www.who.int/news-room/fact-sheets/detail/herpes-simplex-virus
- Juan-Sallés C, Ramos-Vara JA, Prats N, Solé-Nicolás J, Segalés J, Marco AJ. Spontaneous herpes simplex virus infection in common marmosets (Callithrix jacchus). J Vet Diagn Invest. 1997;9:341–5. DOIPubMedGoogle Scholar
- Mätz-Rensing K, Jentsch KD, Rensing S, Langenhuyzen S, Verschoor E, Niphuis H, et al. Fatal Herpes simplex infection in a group of common marmosets (Callithrix jacchus). Vet Pathol. 2003;40:405–11. DOIPubMedGoogle Scholar
- Schrenzel MD, Osborn KG, Shima A, Klieforth RB, Maalouf GA. Naturally occurring fatal herpes simplex virus 1 infection in a family of white-faced saki monkeys (Pithecia pithecia pithecia). J Med Primatol. 2003;32:7–14. DOIPubMedGoogle Scholar
- Lefaux B, Duprez R, Tanguy M, Longeart L, Gessain A, Boulanger E. Nonhuman primates might be highly susceptible to cross-species infectivity by human alpha-herpesviruses. Vet Pathol. 2004;41:302–4. DOIPubMedGoogle Scholar
- Hatt JM, Grest P, Posthaus H, Bossart W. Serologic survey in a colony of captive common marmosets (Callithrix jacchus) after infection with herpes simplex type 1-like virus. J Zoo Wildl Med. 2004;35:387–90. DOIPubMedGoogle Scholar
- Barnes KJ, Garner MM, Wise AG, Persiani M, Maes RK, Kiupel M. Herpes simplex encephalitis in a captive black howler monkey (Alouatta caraya). J Vet Diagn Invest. 2016;28:76–8. DOIPubMedGoogle Scholar
- Costa EA, Luppi MM, Malta MC, Luiz AP, de Araujo MR, Coelho FM, et al. Outbreak of human herpesvirus type 1 infection in nonhuman primates (Callithrix penincillata). J Wildl Dis. 2011;47:690–3. DOIPubMedGoogle Scholar
- Casagrande RA, Pannuti CS, Kanamura C, Freire WS, Grespan A, Matushima ER. Fatal human herpesvirus 1 (HHV-1) infection in captive marmosets (Callithrix jacchus and Callithrix penicillata) in Brazil: clinical and pathological characterization. Pesqui Vet Bras. 2014;34:1109–14. DOIGoogle Scholar
- Imura K, Chambers JK, Uchida K, Nomura S, Suzuki S, Nakayama H, et al. Herpes simplex virus type 1 infection in two pet marmosets in Japan. J Vet Med Sci. 2014;76:1667–70. DOIPubMedGoogle Scholar
- Edwards EE, Birch SM, Hoppes SM, Keating MK, Stoica G. Pathology in Practice. J Am Vet Med Assoc. 2018;253:423–6. DOIPubMedGoogle Scholar
- Huemer HP, Larcher C, Czedik-Eysenberg T, Nowotny N, Reifinger M. Fatal infection of a pet monkey with Human herpesvirus. Emerg Infect Dis. 2002;8:639–42. DOIPubMedGoogle Scholar
- Longa CS, Bruno SF, Pires AR, Romijn PC, Kimura LS, Costa CH. Human herpesvirus 1 in wild marmosets, Brazil, 2008. Emerg Infect Dis. 2011;17:1308–10. DOIPubMedGoogle Scholar
- Bruno SF, Liebhold MM, Mätz-Rensing K, Romao MA, Didier A, Brandes F, et al. [Herpesvirus infections in free living black tufted ear marmosets (Callithrix penicillata, E. Geoffroyi 1812) at the State Park of Serra da Tiririca, Niterói, Rio de Janeiro, Brazil] [in German]. Berl Munch Tierarztl Wochenschr. 1997;110:427–30.PubMedGoogle Scholar
- Martines RB, Ritter JM, Matkovic E, Gary J, Bollweg BC, Bullock H, et al.; COVID-19 Pathology Working Group. Pathology and pathogenesis of SARS-CoV-2 associated with fatal coronavirus disease, United States. Emerg Infect Dis. 2020;26:2005–15. DOIPubMedGoogle Scholar
- Elbadawi LI, Talley P, Rolfes MA, Millman AJ, Reisdorf E, Kramer NA, et al. Non-mumps viral parotitis during the 2014-2015 influenza season in the United States. Clin Infect Dis. 2018;67:493–501. DOIPubMedGoogle Scholar
- Mertz GJ. Asymptomatic shedding of herpes simplex virus 1 and 2: implications for prevention of transmission. J Infect Dis. 2008;198:1098–100. DOIPubMedGoogle Scholar
- Gilbert SC. Oral shedding of herpes simplex virus type 1 in immunocompetent persons. J Oral Pathol Med. 2006;35:548–53. DOIPubMedGoogle Scholar
- Fan S, Cai H, Xu X, Feng M, Wang L, Liao Y, et al. The characteristics of herpes simplex virus type 1 infection in rhesus macaques and the associated pathological features. Viruses. 2017;9:26. DOIPubMedGoogle Scholar
- Emmons RW, Lennette EH. Natural herpesvirus hominis infection of a gibbon (Hylobates lar). Arch Gesamte Virusforsch. 1970;31:215–8. DOIPubMedGoogle Scholar
- Kik MJ, Bos JH, Groen J, Dorrestein GM. Herpes simplex infection in a juvenile orangutan (Pongo pygmaeus pygmaeus). J Zoo Wildl Med. 2005;36:131–4. DOIPubMedGoogle Scholar
- Gilardi KV, Oxford KL, Gardner-Roberts D, Kinani JF, Spelman L, Barry PA, et al. Human herpes simplex virus type 1 in confiscated gorilla. Emerg Infect Dis. 2014;20:1883–6. DOIPubMedGoogle Scholar
- Sehl J, Hölper JE, Klupp BG, Baumbach C, Teifke JP, Mettenleiter TC. An improved animal model for herpesvirus encephalitis in humans. PLoS Pathog. 2020;16:
e1008445 . DOIPubMedGoogle Scholar - Wang X, Li Y, Liu S, Yu X, Li L, Shi C, et al. Direct activation of RIP3/MLKL-dependent necrosis by herpes simplex virus 1 (HSV-1) protein ICP6 triggers host antiviral defense. Proc Natl Acad Sci U S A. 2014;111:15438–43. DOIPubMedGoogle Scholar
- Mootnick AR, Reingold M, Holshuh HJ, Mirkovic RR. Isolation of a herpes simplex virus type 1-like agent from the brain of a mountain agile gibbon (Hylobates agilis agilis) with encephalitis. J Zoo Wildl Med. 1998;29:61–4.PubMedGoogle Scholar
- Kastrukoff LF, Lau AS, Takei F, Smyth MJ, Jones CM, Clarke SR, et al. Redundancy in the immune system restricts the spread of HSV-1 in the central nervous system (CNS) of C57BL/6 mice. Virology. 2010;400:248–58. DOIPubMedGoogle Scholar
- Zimmermann J, Hafezi W, Dockhorn A, Lorentzen EU, Krauthausen M, Getts DR, et al. Enhanced viral clearance and reduced leukocyte infiltration in experimental herpes encephalitis after intranasal infection of CXCR3-deficient mice. J Neurovirol. 2017;23:394–403. DOIPubMedGoogle Scholar
- Whitley RJ, Kimberlin DW. Herpes simplex encephalitis: children and adolescents. Semin Pediatr Infect Dis. 2005;16:17–23. DOIPubMedGoogle Scholar
- Heldstab A, Rüedi D, Sonnabend W, Deinhardt F. Spontaneous generalized Herpesvirus hominis infection of a lowland gorilla (Gorilla gorilla gorilla). J Med Primatol. 1981;10:129–35. DOIPubMedGoogle Scholar
- Landolfi JA, Wellehan JF, Johnson AJ, Kinsel MJ. Fatal human herpesvirus type 1 infection in a white-handed gibbon (Hylobates lar). J Vet Diagn Invest. 2005;17:369–71. DOIPubMedGoogle Scholar
- Lou DI, Kim ET, Meyerson NR, Pancholi NJ, Mohni KN, Enard D, et al. An intrinsically disordered region of the DNA repair protein Nbs1 is a species-specific barrier to herpes simplex virus 1 in primates. Cell Host Microbe. 2016;20:178–88. DOIPubMedGoogle Scholar
- Zhang J, Zhao J, Xu S, Li J, He S, Zeng Y, et al. Species-specific deamidation of cGAS by herpes simplex virus UL37 protein facilitates viral replication. Cell Host Microbe. 2018;24:234–248.e5. DOIPubMedGoogle Scholar
- Clemens SA, Farhat CK. Seroprevalence of herpes simplex 1-2 antibodies in Brazil. [in Portuguese]. Rev Saude Publica. 2010;44:726–34. DOIPubMedGoogle Scholar
- Favoretto SR, de Mattos CC, Morais NB, Alves Araújo FA, de Mattos CA. Rabies in marmosets (Callithrix jacchus), Ceará, Brazil. Emerg Infect Dis. 2001;7:1062–5. DOIPubMedGoogle Scholar
- Sousa DER, Wilson TM, Gonçalves AAB, Ritter JM, Passos PHO, Romano APM, et al. Investigating epizootics: acute fatal toxoplasmosis in urbanized free-ranging black-tufted marmosets (Callithrix penicillata). In: Proceedings: American College of Veterinary Pathology (ACVP) Annual Meeting, 2020 Oct 30–Nov 1. Abstract D-57. Virtual: American College of Veterinary Pathology; 2021.
- de Azevedo Fernandes NCC, Guerra JM, Díaz-Delgado J, Cunha MS, Saad LD, Iglezias SD, et al. Differential yellow fever susceptibility in New World nonhuman primates, comparison with humans, and implications for surveillance. Emerg Infect Dis. 2021;27:47–56. DOIPubMedGoogle Scholar
- Wilson TM, Ritter JM, Martines RB, Gonçalves AAB, Fair P, Galloway R, et al. Pathology and One Health implications of fatal Leptospira interrogans infection in an urbanized, free-ranging, black-tufted marmoset (Callithrix penicillata) in Brazil. Transbound Emerg Dis. 2021;68:3207–16; Epub ahead of print. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: March 08, 2022
Table of Contents – Volume 28, Number 4—April 2022
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Marcio B. Castro, Veterinary Pathology Laboratory, University of Brasília, Via L4 Norte, Campus Universitário Darcy Ribeiro, Asa Norte, Brasília, DF 70910-900, Brazil
Top