Volume 28, Number 4—April 2022
Dispatch
Bordetella hinzii Pneumonia in Patient with SARS-CoV-2 Infection
Cite This Article
Citation for Media
Abstract
Patients infected with severe acute respiratory syndrome coronavirus 2 might have bacterial and fungal superinfections develop. We describe a clinical case of coronavirus disease with pulmonary aspergillosis associated with Bordetella hinzii pneumonia in an immunocompetent patient in France. B. hinzii infections are rare in humans and develop secondary to immunosuppression or debilitating diseases.
Severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) has spread globally and strained health systems with an exponentially increasing number of acute respiratory failures (1). Because severe cases of respiratory distress require ventilator assisted respiration, severe bacterial and fungal co-infections can develop and lead to increased deaths (2,3). Bordetella hinzii is a gram-negative, aerobic coccobacillus initially described as a cause of respiratory infection in poultry and rarely in rodents (4,5). Human infections are rare and occur mostly in immunocompromised persons upon exposure to infected animals (6,7). In humans, these infections were described in 1994 in an HIV-infected patient as a cause of bacteremia (6) and have since been rarely identified in a wide range of infections (8–10). We report a clinical case of SARS-COV-2 infection associated with pulmonary aspergillosis and B. hinzii pneumonia.
This case-patient was identified during routine patient care. Thus, the need for ethics approval was exempted; verbal consent was obtained from the patient.
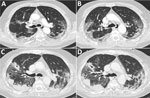
A 63-year-old man with no notable medical history was admitted for cough, asthenia, and shortness of breath starting 3 days before admission. The patient had a positive result for a SARS-CoV-2 rapid antigen autotest. At the emergency department, COVID-19 diagnosis was confirmed by a positive SARS-CoV-2 RT-PCR result for a nasopharyngeal swab sample and chest computed tomography, which showed bilateral ground-glass opacities (50% involvement) and beginning of consolidation in the lower lobes of the lungs (Figure).
He received dexamethasone (6 mg/d for 10 d), subcutaneous, low molecular weight heparin (2 × 6,000 IU/d during the entire hospitalization), ceftriaxone (2 g/d), and spiramycin (1.5 × 106 IU 3×/d). On day 2 of hospitalization, he was transferred to the intensive care unit, antimicrobial drug treatment was stopped, and awake prone positioning was combined with high-flow nasal oxygen therapy.
On day 9, mechanical ventilation was applied because of acute respiratory distress syndrome, worsening hypoxemia, and gas exchange deterioration. There was no documented bacterial superinfection, and after 48 hours, the patient’s oxygenation level had improved.
On day 13, respiratory function worsened; purulent aspiration and fever developed, and inflammatory markers increased (C-reactive protein 254 mg/L [reference <10 mg/L] and procalcitonin 0.35 ng/mL [reference <0.1 ng/mL]). Four-day intravenous piperacillin/tazobactam treatment (4 g/0.5 g 4×/d) was initiated, and an endotracheal aspirate (EA) showed oropharyngeal flora (107 CFU/mL) and 5 × 105 CFU/mL methicillin-susceptible Staphylococcus aureus, 5 × 103 CFU/mL B. hinzii, 5 × 102 CFU/mL amoxicillin-susceptible Escherichia coli, and 5 × 102 CFU/mL Candida tropicalis.
On day 17, another EA showed oropharyngeal flora (107 CFU/mL), decreased methicillin-susceptible S. aureus (5 × 103 CFU/mL), increased B. hinzii (106 CFU/mL), amoxicillin-susceptible E. coli (107 CFU/mL), and Aspergillus fumigatus (102 CFU/mL). A. fumigatus was considered as an infection because of worsening of respiratory failure despite piperacillin/tazobactam treatment, ventilatory support for severe acute respiratory distress syndrome, an A. fumigatus‒positive culture on EA (absent on previous EAs), and a computed tomography scan showing cavitating infiltrates (Figure). Voriconazole treatment (400 mg on the first day, followed by 200 mg/12 h) was given for 21 days, in combination with intravenous co-amoxiclav (1 g 3×/d).
EA was repeated on day 25 because of persistence of fever, progressive clinical deterioration, and worsening of radiologic findings and showed 106 CFU/mL B. hinzii, 105 CFU/mL amoxicillin-susceptible E. coli, and 103 CFU/mL C. tropicalis, which was considered as colonization. We switched treatment to piperacillin/tazobactam (4 g/0.5 g 4×/d for 8 d), which resulted in negative results on subsequent EA samples. Testing of rectal swab samples, blood, and urine cultures remained negative throughout hospitalization. The patient was extubated on day 46 and discharged uneventfully from the hospital.
B. hinzii grew on horse blood agar (bioMérieux, https://www.biomerieux.com) at 37°C after incubation for 24 hours as smooth, gray colonies. We identified B. hinzii by using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Biotyper; Bruker, https://www.bruker.com) and confirmed it by using whole-genome sequencing (Illumina, https://www.illumina.com) as described (11).
We initially performed antimicrobial susceptibility testing by using Etest (bioMérieux), confirmed results by using disk diffusion and broth microdilution (Thermo Fisher Sensititer System; Thermo Fisher, https://www.thermofisher.com) (Table), and interpreted results by using the 2021 European Committee on Antimicrobial Susceptibility Testing pharmacokinetic/pharmacodynamic (nonspecies related) breakpoints (12). B. hinzii CHAR-1 showed resistance to amoxicillin, cefotaxime, aminoglycosides, and ciprofloxacin; intermediate resistance to amoxicillin/clavulanic acid and ceftazidime; and susceptibility to piperacillin/tazobactam, meropenem, and imipenem. New molecules were also tested and remained susceptible, except for ceftolozane/tazobactam (Table).
We identified β-lactamase activity by using B. hinzii CHAR-1 crude protein extracts as described (13). In silico analysis showed a β-lactamase, Hinzii Bordetella lactamase (HBL-1), which had all canonical boxes of a functional broad-spectrum class A penicillinase (13) (Appendix Figure). HBL-1 had 62.7% amino acid identity with BOR-1 β-lactamase from B. parapertussis (13). Highly related sequences (99.7%‒100% amino acid identity) were identified in genome sequences of B. hinzii available in public databases, suggesting that HBL-1–like enzymes might be native to that species (Appendix Figure). MICs of aminopenicillins and carboxypenicillins might be explained by expression of HBL-1, but, as suggested for B. parapertussis, additional nonenzymatic β-lactam resistance mechanisms, such as impermeability, efflux, or penicillin-binding protein affinities, might be associated for B. hinzii. The complete genome and HBL-1 sequences have been deposited in DDBJ/EMBL (accession no. JAJTJL000000000) and GenBank (accession nos. OM212391).
The lung microbiota of deceased patients who had COVID-19 showed complex bacterial and fungal colonization by opportunistic pathogens (14). SARS‐CoV‐2 infection, antimicrobial drug pressure, alveolar damage, persistent lymphocytic depletion, mechanical ventilation, corticosteroid therapy, and prolonged hospital stays might predispose critically ill COVID-19 patients to opportunistic bacterial or fungal superinfection (2,14). Critically ill COVID-19 patients have the highest percentage of secondary pulmonary infections (34.5%) compared with percentages for severely ill (8.3%) and moderately ill (3.9%) COVID-19 patients (15). COVID-19‒associated pulmonary aspergillosis is a worrisome complication in critically ill patients who show increased illnesses and deaths (2). A. fumigatus co-infections are frequent among critically ill COVID-19 patients (2,3). Rothe et al. showed that in a group of 50 critically ill COVID-19 patients admitted to the intensive care unit, 34% were co-infected with Enterobacterales and 18% with A. fumigatus (15).
Human cases of B. hinzii infection are rare and associated mostly with immunosuppression and anterior poultry exposure (7–10). Our patient reported recent exposure to geese and ducks, which probably led to latent or chronic colonization (digestive or respiratory tract) before infection at a time when he was most vulnerable (e.g., COVID-19 and aspergillosis superinfection). Reports of patients who have B. hinzii infections seem to be increasing in recent years, which might reflect emergence of this pathogen or availability of better identification methods, such as matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, 16S rRNA gene sequencing, and whole-genome sequencing (8). Among the few B. hinzii infections described, none reported Aspergillus infections (9).
Reported B. hinzii isolates were frequently multidrug resistant, including resistance to cephalosporins, aminoglycosides, and quinolones, but remained susceptible to piperacillin/tazobactam, ceftazidime, tigecycline, and meropenem (9,10). Interpretation of antimicrobial susceptibility testing is not established, and the choice of antimicrobial drugs and treatment duration are not standardized. Cases with documented pneumonia were successfully treated with piperacillin/tazobactam or cefmetazole (9). Our patient was successfully treated with piperacillin/tazobactam, but treatment with amoxicillin/clavulanic acid failed, probably because of intermediate susceptibility of B. hinzii to this antimicrobial drug. Our study suggests that B. hinzii needs to be taken into account when initiating antimicrobial drug therapy.
Increasing reports of invasive B. hinzii might indicate its emergence as a pathogen in immunocompromised patients. We describe a B. hinzii and A. fumigatus co-infection in a SARS-CoV-2‒infected immunocompetent patient who had no underlying conditions but had probable transient immunosuppression caused by dexamethasone treatment and SARS-CoV-2 infection. Our study highlights the role of opportunistic infections (by fungal or rare bacterial species) in COVID-19 patients and the need to serially monitor the bacteria/fungi in the lower respiratory tract for timely personalized treatment.
Dr. Ben Lakhal is an intensive care physician at Louis Pasteur Hospital, Le Coudray, France. Her research interests include management of severe clinical manifestations of infectious diseases in critical care.
Acknowledgments
We thank Panya Wissa and Emna Warzele for providing helpful discussions and the Institut Pasteur PIBNet for performing whole-genome sequencing of the bacterial isolate.
This study was supported by a grant from the Ministère de l’Education Nationale et de la Recherche (Université Paris-Saclay), Assistance Publique-Hôpitaux de Paris, and the Centre Hospitalier de Chartres.
References
- Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al.; COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1,591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–81. DOIPubMedGoogle Scholar
- Bassetti M, Kollef MH, Timsit JF. Bacterial and fungal superinfections in critically ill patients with COVID-19. Intensive Care Med. 2020;46:2071–4. DOIPubMedGoogle Scholar
- Montrucchio G, Lupia T, Lombardo D, Stroffolini G, Corcione S, De Rosa FG, et al. Risk factors for invasive aspergillosis in ICU patients with COVID-19: current insights and new key elements. Ann Intensive Care. 2021;11:136. DOIPubMedGoogle Scholar
- Register KB, Kunkle RA. Strain-specific virulence of Bordetella hinzii in poultry. Avian Dis. 2009;53:50–4. DOIPubMedGoogle Scholar
- Jiyipong T, Morand S, Jittapalapong S, Raoult D, Rolain JM. Bordetella hinzii in rodents, Southeast Asia. Emerg Infect Dis. 2013;19:502–3. DOIPubMedGoogle Scholar
- Cookson BT, Vandamme P, Carlson LC, Larson AM, Sheffield JV, Kersters K, et al. Bacteremia caused by a novel Bordetella species, “B. hinzii”. J Clin Microbiol. 1994;32:2569–71. DOIPubMedGoogle Scholar
- Fabre A, Dupin C, Bénézit F, Goret J, Piau C, Jouneau S, et al. Opportunistic pulmonary Bordetella hinzii infection after avian exposure. Emerg Infect Dis. 2015;21:2122–6. DOIPubMedGoogle Scholar
- Kattar MM, Chavez JF, Limaye AP, Rassoulian-Barrett SL, Yarfitz SL, Carlson LC, et al. Application of 16S rRNA gene sequencing to identify Bordetella hinzii as the causative agent of fatal septicemia. J Clin Microbiol. 2000;38:789–94. DOIPubMedGoogle Scholar
- Maison-Fomotar M, Sivasubramanian G. Bordetella hinzii pneumonia and bacteremia in a patient with SARS-CoV-2 infection. Emerg Infect Dis. 2021;27:2904–7. DOIPubMedGoogle Scholar
- Collercandy N, Petillon C, Abid M, Descours C, Carvalho-Schneider C, Mereghetti L, et al. Bordetella hinzii: an unusual pathogen in human urinary tract infection. J Clin Microbiol. 2021;59:e02748–20. DOIPubMedGoogle Scholar
- Jousset AB, Bonnin RA, Takissian J, Girlich D, Mihaila L, Cabanel N, et al. Concomitant carriage of KPC-producing and non-KPC-producing Klebsiella pneumoniae ST512 within a single patient. J Antimicrob Chemother. 2020;75:2087–92. DOIPubMedGoogle Scholar
- European Committee on Antimicrobial Susceptibility Testing. PK-PD (non-species related) breakpoint [cited 2022 Jan 31]. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_11.0_Breakpoint_Tables.pdf
- Lartigue MF, Poirel L, Fortineau N, Nordmann P. Chromosome-borne class A BOR-1 beta-Lactamase of Bordetella bronchiseptica and Bordetella parapertussis. Antimicrob Agents Chemother. 2005;49:2565–7. DOIPubMedGoogle Scholar
- Fan J, Li X, Gao Y, Zhou J, Wang S, Huang B, et al. The lung tissue microbiota features of 20 deceased patients with COVID-19. J Infect. 2020;81:e64–7. DOIPubMedGoogle Scholar
- Rothe K, Feihl S, Schneider J, Wallnöfer F, Wurst M, Lukas M, et al. Rates of bacterial co-infections and antimicrobial use in COVID-19 patients: a retrospective cohort study in light of antibiotic stewardship. Eur J Clin Microbiol Infect Dis. 2021;40:859–69. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: March 09, 2022
Table of Contents – Volume 28, Number 4—April 2022
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Hend Ben Lakhal, Service de Réanimation, Centre Hospitalier de Chartres, 4 Rue Claude-Bernard, 28630 Le Coudray, France
Top