Volume 28, Number 6—June 2022
Research
Retrospective Genomic Characterization of a 2017 Dengue Virus Outbreak, Burkina Faso
Figure 6
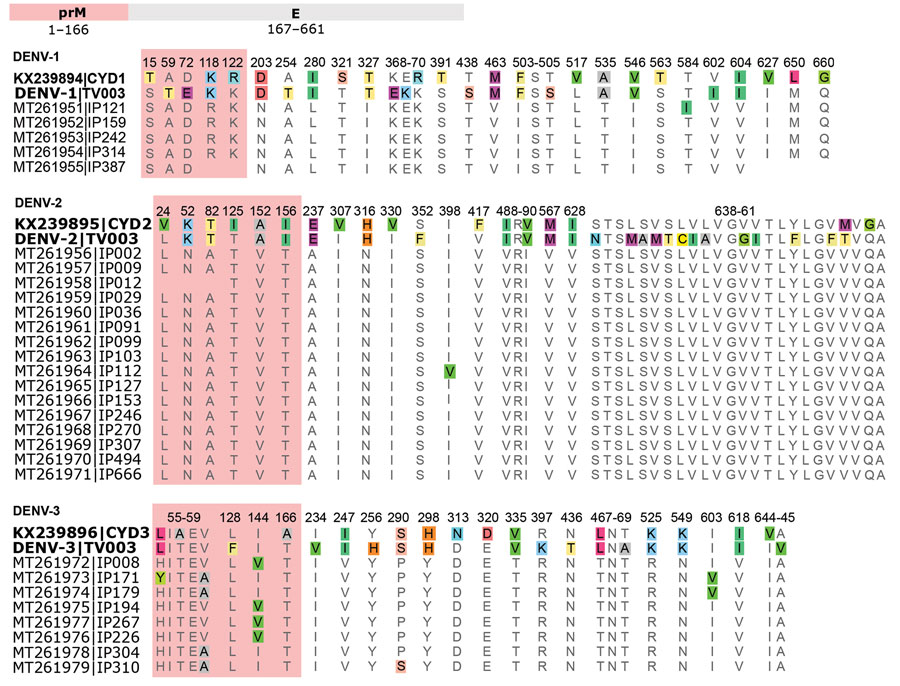
Figure 6. Dengue virus prM and E protein sequence alignments of Dengvaxia and TetraVax-DV-TV003 vaccine strains (boldface) and 2017 Burkina Faso dengue virus outbreak genomes for serotypes 1, 2, and 3. Only amino acid positions with disagreements are shown; single-point disagreements are highlighted. For clarity, prM protein sequences are shaded in red. Numerals represent the prM and E protein amino acid position. CYD, Dengvaxia vaccine; DENV-1, dengue virus serotype 1; DENV-2, dengue virus serotype 2; DENV-3, dengue virus serotype 3; E, envelope; prM, premembrane; TV003, TetraVax-DV-TV003 vaccine.
1These first authors contributed equally to this article.
Page created: March 18, 2022
Page updated: May 22, 2022
Page reviewed: May 22, 2022
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.