Volume 28, Number 7—July 2022
Dispatch
Anncaliia algerae Microsporidiosis Diagnosed by Metagenomic Next-Generation Sequencing, China
Cite This Article
Citation for Media
Abstract
We report a case of Anncaliia algerae microsporidia infection in an immunosuppressed kidney transplant recipient in China. Light microscopy and transmission electron microscopy initially failed to identify A. algerae, which eventually was detected by metagenomic next-generation sequencing. Our case highlights the supporting role of metagenomic sequencing in early identification of uncommon pathogens.
Anncaliia algerae is an uncommon, yet emerging microsporidian parasitic pathogen that can affect immunocompromised patients and cause fatal myositis (1,2). We report a case of A. algerae microsporidiosis, which was initially missed by conventional light microscopy (LM) and subsequent transmission electron microscopy (TEM) of biopsied muscle but eventually identified by metagenomic next-generation sequencing (mNGS).
In March 2021, a 45-year-old male kidney transplant recipient in China was admitted to the hospital for a 2-month history of muscle pain. He was receiving prednisone, tacrolimus, and mycophenolate mofetil for maintenance immunosuppression. The patient did not have respiratory symptoms at admission. Physical examination showed low fever and tenderness and generalized weakness in all 4 limbs. Laboratory investigations revealed serum creatine kinase level within reference range but low CD4+ T lymphocyte count (45 cells/µL; reference range 471–1,220 cells/µL). Serum cytomegalovirus DNA was 1.64 × 102 copies/mL. Results of tests for heavy metals, parasites, and myositis-specific autoantibodies were negative.
The patient was febrile (37.3°C) at admission. Although immunosuppressant drugs were tapered dramatically, and broad-spectrum antimicrobial drugs and ganciclovir were added, the patient remained febrile (Figure 1). Chest computed tomography (CT) imaging showed patchy irregular ground-glass opacity in the left upper lung lobe. Electromyography testing showed myogenic damage in the biceps brachii muscle. Magnetic resonance imaging of lower extremities revealed swollen soft tissue. Bronchoalveolar lavage (BAL) testing was negative for bacteria, fungi, and Pneumocystis jirovecii DNA.
The patient’s myalgia and weakness worsened, his serum creatine kinase level increased (Appendix Figure 1), and watery diarrhea developed. Stool microscopy, gastroduodenoscopy, and colonoscopy revealed no specific abnormalities; repeated chest CT scans showed increased inflammatory exudation and bilateral pleural effusion.
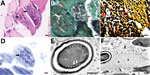
No specific findings were reported from the initial LM of the left biceps brachii biopsy specimen except for degradation and necrosis of myofibers. We performed a second fiberoptic bronchoscopy and sent BAL fluid for untargeted mNGS via NextSeq 550 (Illumina, https://www.illumina.com), which revealed P. jirovecii (142 sequence reads) and A. algerae (127 sequence reads) within 48 hours of receiving the specimen (Appendix Table 1, Figure 2, panel A).
Because the previous biopsy results were negative and we were unfamiliar with A. algerae microsporidia, we performed a literature review and then reviewed the initial muscle biopsy again. We considered the possibility of a combined infection of P. jirovecii and A. algerae, and we consulted an infectious disease specialist who suggested adding oral sulfamethoxazole/trimethoprim (SMZ/TMP; 1,600/320 mg 3×/d), which might be effective against both pathogens. After SMZ/TMP treatment, the patient’s temperature returned to normal for 5 successive days before climbing to 37.8°C on day 43 of admission; we added oral albendazole (400 mg 2×/d) (Figure 1), according to published cases (1,3,4).
However, the patient’s condition continued to deteriorate. On day 51, he decided on comfort care and died 2 days later (Figure 1). On day 52, one day before the patient died, we discovered multiple oval organisms measuring 2–3 µm in scattered clusters under LM in the muscle biopsy sample (Figure 2, panels A–D). After the patient died, we performed mNGS using muscle tissue from the previous biopsy, which yielded 65,311 sequence reads mapped to A. algerae (Appendix Table 2, Figure 2, panel B). A. algerae was confirmed by subsequent PCR testing on muscle tissue, but PCR testing of the remaining BAL specimen yielded no findings because not enough fluid was available in the sample after previous examinations. Eventually, we identified A. algerae via TEM in the third sample section (Figure 1; Figure 2, panels E, F). We deposited the A. algerae sequences into the National Center for Biotechnology Information Sequence Read Archive (accession nos. SRR18339014 for the BAL sample, SRR18339013 for the muscle sample).
A. algerae is a microsporidial species that has been reported to cause human infections since 1999 (5). Of 12 reported cases of human A. algerae infection (1–11), 11 were among immunocompromised patients (Table). Thus, immunodeficiency, as in this patient, appears to be a critical risk factor for A. algerae infection. Although the modes of A. algerae transmission to humans remain uncertain, waterborne transmission, either through ingestion of or exposure to spore-contaminated water, has been postulated as the most likely route (2,4,6). This patient lived near ditches in a rural area of the warm and humid Sichuan Basin and was readily exposed to waters possibly contaminated by A. algerae spores.
A. algerae infection in humans primarily manifests as myositis (1–11), and in reports we reviewed, 5 (62.5%) of 8 case-patients who had A. algerae myositis died (Table). Because of fatality risk, early diagnosis and prompt interventions are crucial. To date, biopsy and microscopy remain the standard approaches in microsporidia identification (12), and the role of mNGS has yet to be confirmed.
Although LM is the fastest diagnostic tool for microsporidiosis, it has several limitations. First, LM is unable to identify the genus and species of microsporidia. Second, the actual turnaround time (5–7 days in our hospital) for LM varies among institutions, which could cause diagnostic delays. Third, the accuracy of LM diagnosis relies on laboratory conditions and microscopist experience. In addition, morphologic features of A. algerae spores overlap with those of other organisms, such as small yeasts, which has led to misdiagnosis under LM (1,11). Thus, familiarity with A. algerae spores and their appearance on histopathology preparations are crucial for rapid diagnosis. In this case, A. algerae spores initially were missed by the microscopist and were detected 2 weeks later during retrospective review because of the relatively long turnaround time.
TEM remains the standard technique for determining the specific microsporidia genus by identifying the ultrastructural characteristics (12). TEM examines a smaller area of tissue at one time but usually has a longer turnaround time than routine LM. TEM results are available in 1–2 days in some institutions, but turnaround time in our hospital takes ≈10–14 days.
As an unbiased, culture-free method capable of detecting all potential pathogens, untargeted mNGS enables identification of unexpected or unknown organisms (13). Compared with hypothesis-driven methods, such as PCR, shotgun mNGS is hypothesis-free, enables survey of all DNA and RNA in multiple samples en masse (13), and generally takes 24–48 hours to produce results. However, mNGS is unlikely to replace conventional diagnostic testing because of its limitations, such as high cost (US $522 for DNA detection and $894 for both DNA and RNA in our hospital), lack of a unified workflow, and no standard methods for interpreting results (13). Instead, mNGS can serve as a valuable adjunct tool in diagnosing uncommon or unexplained infections when conventional methods such as LM fail.
Albendazole and fumagillin have been used to treat A. algerae infections in previously reported cases (Table). We have easy access to albendazole, but no access to fumagillin. SMZ/TMP was reported to have no effect against Enterocytozoon bieneusi microsporidiosis (14), but data regarding effectiveness against A. algerae microsporidia were limited. Treatment was greatly delayed in this patient because of our lack of clinical experience with A. algerae microsporidia and the late microscopy findings. Early treatment, along with minimized immunosuppression, might be crucial for the successful management of A. algerae infection (1,3,4).
In conclusion, A. algerae microsporidia infection requires early diagnosis and prompt intervention. LM alone cannot identify microsporidia genus and species; thus, TEM or genomic sequencing are needed for correct diagnosis. As a sensitive, culture-independent approach, mNGS could be a promising adjunct tool for the early identification of uncommon pathogens, such as A. algerae and other microsporidia.
Mr. Liu is a doctoral student at the Department of Nephrology and Kidney Research Institute, West China Hospital of Sichuan University, Chengdu, China. His research interest is infections in renal transplant patients.
Acknowledgments
We thank Song Lei for providing professional help in the transmission electron microscopy of A. algerae spores in muscle tissue. We also thank Teng Xu and Huan Xu for providing the mNGS methods, interpreting the mNGS results, and performing PCR tests.
This work was supported by the National Natural Science Foundation of China (grant no. 81771714) and 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (grant no. ZYJC18004).
References
- Watts MR, Chan RCF, Cheong EYL, Brammah S, Clezy KR, Tong C, et al. Anncaliia algerae microsporidial myositis. Emerg Infect Dis. 2014;20:185–91. DOIPubMedGoogle Scholar
- Coyle CM, Weiss LM, Rhodes LV III, Cali A, Takvorian PM, Brown DF, et al. Fatal myositis due to the microsporidian Brachiola algerae, a mosquito pathogen. N Engl J Med. 2004;351:42–7. DOIPubMedGoogle Scholar
- Boileau M, Ferreira J, Ahmad I, Lavallée C, Qvarnstrom Y, Dufresne SF. Successful treatment of disseminated Anncaliia algerae microsporidial infection with combination fumagillin and albendazole. Open Forum Infect Dis. 2016;3:
ofw158 . DOIPubMedGoogle Scholar - Sutrave G, Maundrell A, Keighley C, Jennings Z, Brammah S, Wang M-X, et al. Anncaliia algerae microsporidial myositis, New South Wales, Australia. Emerg Infect Dis. 2018;24:1528–31. DOIPubMedGoogle Scholar
- Visvesvara GS, Belloso M, Moura H, Da Silva AJ, Moura IN, Leitch GJ, et al. Isolation of Nosema algerae from the cornea of an immunocompetent patient. J Eukaryot Microbiol. 1999;46:10S.PubMedGoogle Scholar
- Ziad F, Robertson T, Watts MR, Copeland J, Chiu G, Wang D, et al. Fatal disseminated Anncaliia algerae myositis mimicking polymyositis in an immunocompromised patient. Neuromuscul Disord. 2021;31:877–80. DOIPubMedGoogle Scholar
- Visvesvara GS, Moura H, Leitch GJ, Schwartz DA, Xiao LX. Public health importance of Brachiola algerae (Microsporidia)—an emerging pathogen of humans. Folia Parasitol (Praha). 2005;52:83–94. DOIPubMedGoogle Scholar
- Cali A, Neafie R, Weiss LM, Ghosh K, Vergara RB, Gupta R, et al. Human vocal cord infection with the Microsporidium Anncaliia algerae. J Eukaryot Microbiol. 2010;57:562–7. DOIPubMedGoogle Scholar
- Field AS, Paik JY, Stark D, Qiu MR, Morey A, Plit ML, et al. Myositis due to the microsporidian Anncaliia (Brachiola) algerae in a lung transplant recipient. Transpl Infect Dis. 2012;14:169–76. DOIPubMedGoogle Scholar
- Chacko B, Trevillian P. Microsporidial myositis in a kidney transplant recipient [abstract 82]. In: Program and abstracts of Annual Scientific Meeting Transplantation Society of Australia and New Zealand. Canberra (ACT, Australia): Transplantation Society of Australia and New Zealand; 2013. p. 96.
- Anderson NW, Muehlenbachs A, Arif S, Bruminhent J, Deziel PJ, Razonable RR, et al. A fatal case of disseminated microsporidiosis due to Anncaliia algerae in a renal and pancreas allograft recipient. Open Forum Infect Dis. 2019;6:
ofz285 . DOIPubMedGoogle Scholar - Franzen C, Müller A. Microsporidiosis: human diseases and diagnosis. Microbes Infect. 2001;3:389–400. DOIPubMedGoogle Scholar
- Costa SF, Weiss LM. Drug treatment of microsporidiosis. Drug Resist Updat. 2000;3:384–99. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: June 06, 2022
1These first authors contributed equally to this article.
Table of Contents – Volume 28, Number 7—July 2022
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Yun-Ying Shi, Department of Nephrology, West China Hospital of Sichuan University, No. 37 Guoxue Alley Wuhou District, Chengdu 610041, China
Top