Volume 28, Number 7—July 2022
Dispatch
Use of Human Intestinal Enteroids to Evaluate Persistence of Infectious Human Norovirus in Seawater
Figure 1
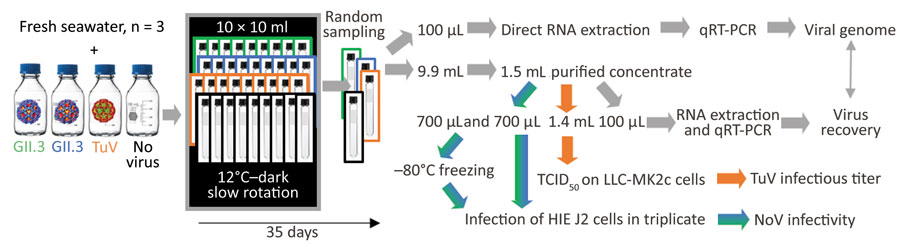
Figure 1. Study design on use of HIEs to evaluate persistence of infectious human norovirus in seawater. Comparison of the stability of 2 human norovirus strains (GII.3 indicated by green, GII.4 indicated by blue) and TuV (orange) in seawater. We conducted 3 independent experiments with different fresh seawater samples. Spiked seawater (120 mL) was split in 10 mL aliquots in glass tubes, incubated at 12°C in the dark under constant rotation (10 rpm), and randomly sampled once or twice per week for 5 weeks (35 days). Grey arrows indicate steps or treatments applied to all samples; blue-green arrows indicate steps or treatments applied to human norovirus and control without virus; orange arrows indicate steps or treatments applied to TuV only. HIE, human intestinal enteroid, NoV, norovirus; qRT-PCR, one-step quantitative reverse transcription PCR; TCID50, 50% median tissue culture infectious dose; TuV, Tulane virus.
1These first authors contributed equally to this article.
2Current affiliation: Centro de Investigaciones Biologicas–Facultade de Bioloxía & CRETUS, Universidade de Santiago de Compostela, Santiago de Compostela, Spain.
3Current affiliation: SECALIM UMR 104 Oniris/Inrae, Nantes, France.