Volume 29, Number 10—October 2023
Research
Posttransfusion Sepsis Attributable to Bacterial Contamination in Platelet Collection Set Manufacturing Facility, United States
Figure 1
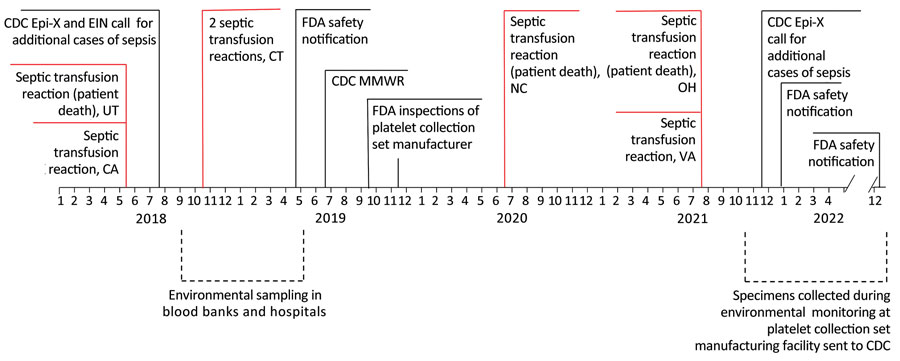
Figure 1. Investigation timeline of transfusion-transmitted sepsis cases and key events for bacterial contamination in platelet collection set manufacturing facilities, United States, 2018–2022. CDC, Centers for Disease Control and Prevention; EIN, Emerging Infections Network; Epi-X, Epidemic Information Exchange; FDA, Food and Drug Administration; MMWR, report published in Morbidity and Mortality Weekly Report (9).
References
- Wagner SJ, Friedman LI, Dodd RY. Transfusion-associated bacterial sepsis. Clin Microbiol Rev. 1994;7:290–302. DOIPubMedGoogle Scholar
- Eder AF, Kennedy JM, Dy BA, Notari EP, Weiss JW, Fang CT, et al.; American Red Cross Regional Blood Centers. Bacterial screening of apheresis platelets and the residual risk of septic transfusion reactions: the American Red Cross experience (2004-2006). Transfusion. 2007;47:1134–42. DOIPubMedGoogle Scholar
- Kuehnert MJ, Roth VR, Haley NR, Gregory KR, Elder KV, Schreiber GB, et al. Transfusion-transmitted bacterial infection in the United States, 1998 through 2000. Transfusion. 2001;41:1493–9. DOIPubMedGoogle Scholar
- Free RJ, Sapiano MRP, Chavez Ortiz JL, Stewart P, Berger J, Basavaraju SV. Continued stabilization of blood collections and transfusions in the United States: findings from the 2021 National Blood Collection and Utilization Survey. Transfusion. 2023;trf.17360.
- Hong H, Xiao W, Lazarus HM, Good CE, Maitta RW, Jacobs MR. Detection of septic transfusion reactions to platelet transfusions by active and passive surveillance. Blood. 2016;127:496–502. DOIPubMedGoogle Scholar
- Fenwick AJ, Gehrie EA, Marshall CE, Tobian AAR, Shrestha R, Kacker S, et al. Secondary bacterial culture of platelets to mitigate transfusion-associated sepsis: A 3-year analysis at a large academic institution. Transfusion. 2020;60:2021–8. DOIPubMedGoogle Scholar
- Brecher ME, Hay SN. Bacterial contamination of blood components. Clin Microbiol Rev. 2005;18:195–204. DOIPubMedGoogle Scholar
- US Food and Drug Administration. Bacterial risk control strategies for blood collection establishments and transfusion services to enhance the safety and availability of platelets for transfusion. 2020 [cited 2023 Aug 2]. https://www.fda.gov/media/123448/download
- Jones SA, Jones JM, Leung V, Nakashima AK, Oakeson KF, Smith AR, et al. Sepsis attributed to bacterial contamination of platelets associated with a potential common source—multiple states, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:519–23. DOIPubMedGoogle Scholar
- US Food and Drug Administration. Important information for blood establishments and transfusion services regarding bacterial contamination of platelets for transfusion. 2021 [cited 2023 Aug 2]. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/important-information-blood-establishments-and-transfusion-services-regarding-bacterial
- US Food and Drug Administration. Important information for blood establishments and transfusion services regarding bacterial contamination of platelets for transfusion. 2022 [cited 2023 Aug 2]. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/important-information-blood-establishments-and-transfusion-services-regarding-bacterial-0
- Stanton RA, Vlachos N, de Man TJ, Lawsin A, Halpin AL. Development and application of QuAISAR-H: a bioinformatics pipeline for short read sequences of healthcare-associated pathogens. Presented at: ASM Conference on Rapid Applied Microbial Next Generation Sequencing and Bioinformatics Pipelines; Tyson Falls, VA, USA; September 25, 2018.
- Nevala-Plagemann C, Powers P, Mir-Kasimov M, Rose R. A fatal case of septic shock secondary to Acinetobacter bacteremia acquired from a platelet transfusion. Case Rep Med. 2019;2019:
3136493 . DOIPubMedGoogle Scholar - Fridey JL, Stramer SL, Nambiar A, Moayeri M, Bakkour S, Langelier C, et al. Sepsis from an apheresis platelet contaminated with Acinetobacter calcoaceticus/baumannii complex bacteria and Staphylococcus saprophyticus after pathogen reduction. Transfusion. 2020;60:1960–9. DOIPubMedGoogle Scholar
- Fadeyi EA, Wagner SJ, Goldberg C, Lu T, Young P, Bringmann PW, et al. Fatal sepsis associated with a storage container leak permitting platelet contamination with environmental bacteria after pathogen reduction. Transfusion. 2021;61:641–8. DOIPubMedGoogle Scholar
- Food and Drug Administration. FDA dashboards. 2023 [cited 2023 Jan 31]. https://datadashboard.fda.gov/ora/firmprofile.htm?FEIi=2627511
- Eder AF, Dy BA, DeMerse B, Wagner SJ, Stramer SL, O’Neill EM, et al. Apheresis technology correlates with bacterial contamination of platelets and reported septic transfusion reactions. Transfusion. 2017;57:2969–76. DOIPubMedGoogle Scholar
- Perez P, Salmi LR, Folléa G, Schmit JL, de Barbeyrac B, Sudre P, et al.; BACTHEM Group; French Haemovigilance Network. Determinants of transfusion-associated bacterial contamination: results of the French BACTHEM Case-Control Study. Transfusion. 2001;41:862–72. DOIPubMedGoogle Scholar
- LaVerda D, Shinefeld L, Best N, Lisitu J, Tambolleo G, Vallejo YR. Evaluation of an improved rapid bacterial assay with untreated and pathogen-reduced platelets: Detection of Acinetobacter strains. Transfusion. 2021;61:2710–7. DOIPubMedGoogle Scholar
- Kundrapu S, Srivastava S, Good CE, Lazarus HM, Maitta RW, Jacobs MR. Bacterial contamination and septic transfusion reaction rates associated with platelet components before and after introduction of primary culture: experience at a US Academic Medical Center 1991 through 2017. Transfusion. 2020;60:974–85. DOIPubMedGoogle Scholar
- Haass KA, Sapiano MRP, Savinkina A, Kuehnert MJ, Basavaraju SV. Transfusion-transmitted infections reported to the national healthcare safety network hemovigilance module. Transfus Med Rev. 2019;33:84–91. DOIPubMedGoogle Scholar
- Heltberg O, Skov F, Gerner-Smidt P, Kolmos HJ, Dybkjaer E, Gutschik E, et al. Nosocomial epidemic of Serratia marcescens septicemia ascribed to contaminated blood transfusion bags. Transfusion. 1993;33:221–7. DOIPubMedGoogle Scholar
- Cloutier M, De Korte D; ISBT Transfusion-Transmitted Infectious Diseases Working Party, Subgroup on Bacteria. Residual risks of bacterial contamination for pathogen-reduced platelet components. Vox Sang. 2022;117:879–86. DOIPubMedGoogle Scholar
- Gammon RR, Reik RA, Stern M, Vassallo RR, Waxman DA, Young PP, et al. Acquired platelet storage container leaks and contamination with environmental bacteria: A preventable cause of bacterial sepsis. Transfusion. 2022;62:641–50. DOIPubMedGoogle Scholar
- Crawford E, Kamm J, Miller S, Li LM, Caldera S, Lyden A, et al. Investigating transfusion-related sepsis using culture-independent metagenomic sequencing. Clin Infect Dis. 2020;71:1179–85. DOIPubMedGoogle Scholar
- Wong D, Nielsen TB, Bonomo RA, Pantapalangkoor P, Luna B, Spellberg B. Clinical and pathophysiological overview of Acinetobacter infections: a century of challenges. Clin Microbiol Rev. 2017;30:409–47. DOIPubMedGoogle Scholar
- Lawal OU, Fraqueza MJ, Bouchami O, Worning P, Bartels MD, Gonçalves ML, et al. Foodborne origin and local and global spread of Staphylococcus saprophyticus causing human urinary tract infections. Emerg Infect Dis. 2021;27:880–93. DOIPubMedGoogle Scholar
- Lawal OU, Barata M, Fraqueza MJ, Worning P, Bartels MD, Goncalves L, et al. Staphylococcus saprophyticus from clinical and environmental origins have distinct biofilm composition. Front Microbiol. 2021;12:
663768 . DOIPubMedGoogle Scholar - Gedefie A, Demsis W, Ashagrie M, Kassa Y, Tesfaye M, Tilahun M, et al. Acinetobacter baumannii biofilm formation and its role in disease pathogenesis: a review. Infect Drug Resist. 2021;14:3711–9. DOIPubMedGoogle Scholar
- Kerantzas CA, Merwede J, Snyder EL, Hendrickson JE, Tormey CA, Kazmierczak BI, et al. Assessment of polymicrobial interactions in bacterial isolates from transfused platelet units associated with sepsis. Transfusion. 2022;62:2458–63. DOIPubMedGoogle Scholar
- Greco-Stewart VS, Brown EE, Parr C, Kalab M, Jacobs MR, Yomtovian RA, et al. Serratia marcescens strains implicated in adverse transfusion reactions form biofilms in platelet concentrates and demonstrate reduced detection by automated culture. Vox Sang. 2012;102:212–20. DOIPubMedGoogle Scholar
- Jacobs MR, Good CE, Lazarus HM, Yomtovian RA. Relationship between bacterial load, species virulence, and transfusion reaction with transfusion of bacterially contaminated platelets. Clin Infect Dis. 2008;46:1214–20. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. FY23 broad agency announcement notice 75D301–23-R-72545. 2023 [cited 2023 Jan 31]. https://sam.gov/opp/15229982f7c348f69fd35e9a0add8aba/view
Page created: August 02, 2023
Page updated: September 20, 2023
Page reviewed: September 20, 2023
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.