Circovirus Hepatitis Infection in Heart-Lung Transplant Patient, France
Philippe Pérot
1, Jacques Fourgeaud
1, Claire Rouzaud
1, Béatrice Regnault, Nicolas Da Rocha, Hélène Fontaine, Jérôme Le Pavec, Samuel Dolidon, Margaux Garzaro, Delphine Chrétien, Guillaume Morcrette, Thierry Jo Molina, Agnès Ferroni, Marianne Leruez-Ville, Olivier Lortholary
2, Anne Jamet
2, and Marc Eloit
2
Author affiliations: Institut Pasteur Pathogen Discovery Laboratory, Paris, France (P. Pérot, B. Regnault, N. Da Rocha, D. Chrétien, M. Eloit); The OIE Collaborating Center for the Detection and Identification in Humans of Emerging Animal Pathogens, Paris (P. Pérot, B. Regnault, N. Da Rocha, D. Chrétien, M. Eloit); Institut Imagine, Paris (J. Fourgeaud, M. Leruez-Ville); Université Paris Cité, Paris (J. Fourgeaud, A. Jamet); Necker-Enfants Malades Hospital, Paris (J. Fourgeaud, G. Morcrette, T.J. Molina, A. Ferroni, M. Leruez-Ville, A. Jamet); Hôpital Necker Enfants-Malades Centre d'Infectiologie Necker-Pasteur, Paris (C. Rouzaud, M. Garzaro, O. Lortholary); Groupe Hospitalier Paris Saint Joseph-Marie Lannelongue, Équipe Mobile de Microbiologie Clinique, Paris (C. Rouzaud); Hôpital Cochin Département d'Hépatologie-Addictologie, Paris (H. Fontaine); Université Paris–Sud, Paris (J. Le Pavec); Hôpital Marie Lannelongue Service de Pneumologie et Transplantation Pulmonaire, Le Plessis-Robinson, France (J. Le Pavec, S. Dolidon); Institut Necker Enfants Malades, Paris (A. Jamet); Ecole Nationale Vétérinaire d’Alfort, Maisons-Alfort, France (M. Eloit)
Main Article
Figure 1
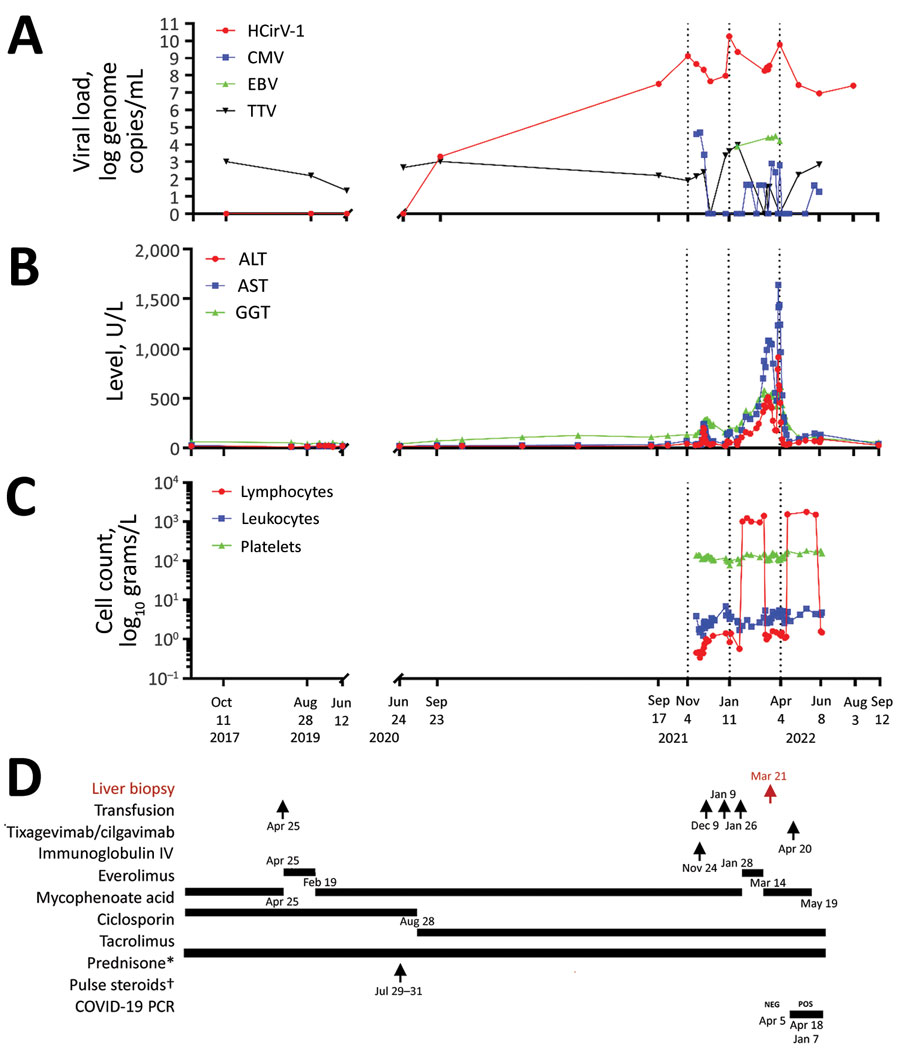
Figure 1. Clinical and laboratory data over time for a heart-lung transplant patient in France who had cytolytic hepatitis caused by HCirV-1 develop. A–C) Monitoring of patient over time: A) viral loads; B) liver cytolysis markers; C) blood cell counts. D) Patient’s treatment history; red indicates timing of liver biopsy (March 21, 2022). Average values are depicted for HCirV-1 and TTV viral loads in plasma and blood on June 8 and August 3, 2022. *Dose 5mg/day; †solumedrol dose 500 mg/day. ALT, alanine transaminase; AST, aspartate aminotransferase; CMV, cytomegalovirus; EBV, Epstein-Barr virus; GGT, gamma-glutamyl transferase; HCirV-1, human circovirus type 1; IV, intravenous; TTV, Torque teno virus.
Main Article
Page created: December 06, 2022
Page updated: January 23, 2023
Page reviewed: January 23, 2023
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.
