Volume 29, Number 4—April 2023
Dispatch
Serial Interval and Incubation Period Estimates of Monkeypox Virus Infection in 12 Jurisdictions, United States, May–August 2022
Cite This Article
Citation for Media
Abstract
Using data from 12 US health departments, we estimated mean serial interval for monkeypox virus infection to be 8.5 (95% credible interval 7.3–9.9) days for symptom onset, based on 57 case pairs. Mean estimated incubation period was 5.6 (95% credible interval 4.3–7.8) days for symptom onset, based on 35 case pairs.
Since May 6, 2022, mpox (formerly monkeypox) cases have been reported across the globe. According to the Centers for Disease Control and Prevention (CDC), 85,115 confirmed mpox cases and 182 deaths have occurred in 110 locations across historically endemic and nonendemic regions as of January 25, 2023 (1). Mpox symptoms usually start within 3 weeks of exposure to monkeypox virus (MPXV) and may include fever, headache, chills, swollen lymph nodes, and exhaustion (2). A rash usually develops within 1–4 days after onset of symptoms. MPXV is transmitted through close contact with infectious rash, scabs, or body fluids; respiratory droplets during prolonged face-to-face contact; and fomites such as clothing, towels, or bedding (1). Transmission in the current outbreak has occurred primarily through close physical contact associated with sexual activities among gay, bisexual, and other men who have sex with men. Transmission of MPXV is possible from the time of symptom onset until all scabs have fallen off and fully healed (3).
The serial interval is defined as the time between symptom onset in a primary case-patient and symptom onset in the secondary case-patient and depends on the incubation period (the time from a person’s infection to the onset of signs and symptoms) (4), epidemic phase, and population contact patterns. The serial interval is critical for estimating the effective reproduction number (Rt) and forecasting incidence, both of which are important for understanding the course of an outbreak and the effect of interventions (e.g., antiviral drugs and vaccines). In the current outbreak, many patients report multiple anonymous sex partners or attendance at large events, such as festivals, in the 3 weeks before symptom onset, which has complicated efforts to identify primary and secondary case pairs. By using preliminary data from 17 mpox case pairs in the United Kingdom, researchers estimated the mean serial interval to be 9.8 days with high uncertainty (95% credible interval [CrI] 5.9–21.4 days) (5). An investigation of 16 primary and secondary case pairs in Italy indicated the estimated mean generation time, or time between infection of primary and secondary cases, to be 12.5 (95% CrI 7.5–17.3) days (6). In this report, we estimate the serial interval and incubation period for symptom onset and rash onset for MPXV infection in the United States.
Data on self-reported symptom and rash onset dates for primary and secondary case pairs, including the type of contact that occurred between pairs, were compiled by 12 state and local health departments. We examined serial interval for both earliest symptom onset and rash onset because the latter may be more specific to mpox than the other signs. Earliest symptom onset included any mpox symptom as defined by CDC (2), including rash. We only included cases if there was a high degree of certainty that the secondary case-patient was infected by the primary case-patient (Appendix).
For each case pair, we calculated days between onset of any mpox symptoms and days between rash onset in the primary and secondary case-patients. We used the EpiEstim package version 4.1.2 in R software (The R Foundation for Statistical Computing, https://www.r-project.org) to estimate the distribution of the serial interval for known primary and secondary case pairs using Bayesian methods for symptom and rash onset (Appendix) (7). We did not adjust for right-truncation of the data because we included cases during the stable or declining phase of the outbreak (8).
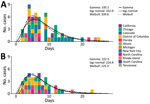
We received data for 120 case pairs from 13 jurisdictions (Appendix Figures 1, 2). Fifty-seven case pairs met the inclusion criteria (Figure 1; Appendix). Dates of symptom onset among primary case-patients ranged from May 11 to August 13, 2022 (Appendix Figure 3). Forty of the 57 pairs included rash onset dates for primary and secondary case-patients. We also considered in our analysis the type of contact for the case pairs (Appendix Table 1). The gamma distribution provided the best fit to the serial interval data. The overall mean estimated serial interval for symptom onset was 8.5 (95% CrI 7.3–9.9) days (SD 5.0 [95% CrI 4.0–6.4] days) and for rash onset was 7.0 (95% CrI 5.8–8.4) days (SD 4.2 [95% CrI 3.2–5.6] days) (Table; Appendix Tables 2, 3).
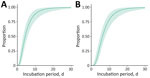
We estimated the incubation period using 22 US mpox cases reported in an earlier study (K. Charniga et al., unpub. data, https://doi.org/10.1101/2022.06.22.22276713) plus 14 cases from our dataset (Appendix). Of the new case-patients, 10 were exposed during a single day. The mean incubation period from exposure to symptom onset for 36 case-patients was 5.6 (95% CrI 4.3–7.8) days (SD 4.4 [95% CrI 2.8–8.7] days), whereas the mean incubation period from exposure to rash onset for 35 case-patients was 7.5 (95% CrI 6.0–9.8) days (SD 4.9 [95% CrI 3.2–8.8] days) (Figure 2; Appendix Figure 4).
Determining the serial interval of a pathogen can inform our understanding of the timing of transmission relative to symptom onset. The serial interval of 8.5 days for symptom onset was similar to an estimate from the United Kingdom of 9.8 days after correcting for right-truncation (5) but shorter than the generation time of 12.5 days reported from Italy (6). The serial interval correlates with human behavior and can decrease with increasing awareness among men who have sex with men or interventions, a pattern similar to that observed among the general population during the COVID-19 pandemic (9). The Italy estimates were from the initial period of the epidemic (May–June 2022), whereas our study was for July–August 2022. Serial interval changes can be very rapid (10). We also found a serial interval of 7.0 days for rash onset. The estimated serial interval for symptom onset was longer than that for the incubation period (5.6 days), suggesting most transmission occurred after the onset of symptoms in the primary case-patient. Conversely, the serial interval for rash onset (7.0 days) was slightly shorter than that for the rash incubation period (7.5 days), which may suggest some prerash transmission; indeed, there were instances in the observed data where secondary case-patients were exposed before onset of reported rash in the primary case-patient. However, the credible intervals for the estimates overlap. The serial interval for symptom onset ranged from 2 to 25 days. This wide range may be attributable in part to variability in the nature and intensity of contact.
The first limitation of this study is that precise ascertainment of symptom and rash onset dates is critical for serial interval estimation, but initial mpox symptoms are often nonspecific and may be unrelated to MPXV infection. Second, despite careful selection of linked primary and secondary case pairs, exposure from additional unknown sources may have occurred. Third, social desirability bias may have factored into the self-reported exposures before infection. Fourth, serial interval may vary by age, underlying conditions, vaccination status, or contact type (route of exposure); we did not stratify our analysis by these factors because of limited data. Fifth, we excluded secondary case-patients who had symptom onset on the same day as or before the primary case-patient to ensure a high degree of confidence linking case pairs; however, the serial interval could be negative (11). Sixth, the serial interval for rash onset could be biased if rash is more quickly identified in the secondary case-patient because of case finding and investigation of the primary case-patient.
Notwithstanding those limitations, our estimate of the serial interval for MPXV infection includes more case pairs than have been reported previously from the United Kingdom (5) and Italy (6). We also provide estimates for rash onset, which may be more reliable than initial symptom onset for determining serial interval.
Dr. Madewell is a fellow in the Public Health Analytics and Modeling Track of the CDC Steven M. Teustch Prevention Effectiveness Fellowship. His primary research interests include epidemiologic study and modeling of infectious diseases. Dr. Charniga is a fellow in the Public Health Analytics and Modeling Track of the CDC Steven M. Teustch Prevention Effectiveness Fellowship. Her primary research interests include outbreak analysis and zoonotic diseases.
2022 Mpox Outbreak Response Team, by affiliation: CDC (Julia Shaffner); California Department of Public Health (Shua J. Chai, Marisa A.P. Donnelly, Robert E. Snyder, Cameron Stainken, Eric C. Tang, Akiko Kimura, Jason Robert C. Singson, and Philip Peters); Colorado Department of Public Health and Environment (Robyn Weber and Erin Youngkin); District of Columbia Department of Health (Sarah Gillani, Karla Miletti, and Allison Morrow); Tennessee Department of Health (Caleb Wiedeman); Florida Department of Health (Danielle Stanek and Joshua Moore); Chicago Department of Public Health (Bridget Brassil, Isaac Ghinai, Janna Kerins, Aaron Krusniak, Sarah Love, Peter Ruestow, and Emma Weber); North Carolina Department of Health and Human Services (Erin Ricketts); Rhode Island Department of Health (Emma Creegan, Karen Luther, and Patricia McAuley); Will County Health Department Mpox Investigation Team; Kendall County Health Department Mpox Investigation Team.
This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy (45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C. Sect. 241(d); 5 U.S.C. Sect. 552a; 44 U.S.C. Sect. 3501 et seq).
Acknowledgment
We thank mpox response teams from state and local health departments in the following jurisdictions: California, Chicago, Colorado, District of Columbia, Hawaii, Florida, Illinois, Michigan, New York City, North Carolina, Rhode Island, South Carolina, and Tennessee. Hawaii contributed data, but the case pairs did not meet the inclusion criteria. We also thank the CDC Center for Forecasting and Outbreak Analytics for technical assistance, the Mpox Task Force in the CDC Center for State, Tribal, Local, and Territorial Support for outreach and liaison, and the Data, Analytics, and Visualization Task Force Informatics Team, CDC, for data management and support.
References
- Centers for Disease Control and Prevention. Monkeypox. 2022 [cited 2022 Sep 19]. https://www.cdc.gov/poxvirus/monkeypox
- Centers for Disease Control and Prevention. Monkeypox: signs and symptoms. 2022 [cited 2022 Nov 22]. https://www.cdc.gov/poxvirus/monkeypox/symptoms/index.html
- Delaney KP, Sanchez T, Hannah M, Edwards OW, Carpino T, Agnew-Brune C, et al. Strategies adopted by gay, bisexual, and other men who have sex with men to prevent monkeypox virus transmission—United States, August 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1126–30. DOIPubMedGoogle Scholar
- Reynolds MG, Yorita KL, Kuehnert MJ, Davidson WB, Huhn GD, Holman RC, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194:773–80. DOIPubMedGoogle Scholar
- UK Health Security Agency. Investigation into monkeypox outbreak in England: technical briefing 1. 2022 [cited 2022 Aug 25]. https://www.gov.uk/government/publications/monkeypox-outbreak-technical-briefings/investigation-into-monkeypox-outbreak-in-england-technical-briefing-1#:~:text=Without%20correcting%20for%20right%2Dtruncation,8.9)%2C%20see%20Figure%202a
- Guzzetta G, Mammone A, Ferraro F, Caraglia A, Rapiti A, Marziano V, et al. Early estimates of monkeypox incubation period, generation time, and reproduction number, Italy, May–June 2022. Emerg Infect Dis. 2022;28:2078–81. DOIPubMedGoogle Scholar
- Cori A, Cauchemez S, Ferguson NM, Fraser C, Dahlqwist E, Demarsh PA, et al. EpiEstim: estimate time varying reproduction numbers from epidemic curves. 2020 [cited 2022 Nov 23]. https://cran.r-project.org/web/packages/EpiEstim/index.html
- Ward T, Christie R, Paton RS, Cumming F, Overton CE. Transmission dynamics of monkeypox in the United Kingdom: contact tracing study. BMJ. 2022;379:
e073153 . DOIPubMedGoogle Scholar - Ali ST, Wang L, Lau EHY, Xu X-K, Du Z, Wu Y, et al. Serial interval of SARS-CoV-2 was shortened over time by nonpharmaceutical interventions. Science. 2020;369:1106–9. DOIPubMedGoogle Scholar
- Xin H, Wang Z, Feng S, Sun Z, Yu L, Cowling BJ, et al. Transmission dynamics of SARS-CoV-2 Omicron variant infections in Hangzhou, Zhejiang, China, January-February 2022. Int J Infect Dis. 2023;126:132–5. DOIPubMedGoogle Scholar
- Du Z, Xu X, Wu Y, Wang L, Cowling BJ, Meyers LA. Serial interval of COVID-19 among publicly reported confirmed cases. Emerg Infect Dis. 2020;26:1341–3. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: March 02, 2023
1These first authors contributed equally to this article.
2Additional members of 2022 Mpox Outbreak Response Team who contributed data are listed at the end of this article.
Table of Contents – Volume 29, Number 4—April 2023
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Zachary J. Madewell, Centers for Disease Control and Prevention, 1600 Clifton Rd NE, Mailstop H21-9, Atlanta, GA 30329-4027, USA
Top