Volume 31, Number 9—September 2025
Research Letter
Monkeypox Virus Clade IIa Infections, Liberia, 2023–2024
Cite This Article
Citation for Media
Abstract
We performed monkeypox virus genome sequencing on clinical samples from Liberia, yielding 5 clade IIa genomes. Our analysis found no evidence of sustained human-to-human transmission, suggesting independent zoonotic spillovers from a diverse viral lineage. Public health officials should continue monitoring and sequencing efforts to identify emerging monkeypox virus lineages.
Monkeypox virus (MPXV; Poxviridae: Orthopoxvirus monkeypox) isolates cluster into 2 major clades, I and II, and each has subclades a and b (1,2). Clades Ia and IIa, primarily circulating in Equatorial Africa, generally cause zoonotic spillovers, whereas specific lineages of clades Ib and IIb from the 2024 outbreak in Central Africa have been associated with sustained human-to-human transmission (3,4). Clade I typically causes more severe disease and higher case-fatality rates than clade II, and the 2024 outbreaks in Democratic Republic of the Congo showed lower case-fatality rates for clade Ia and less-severe clade Ib infections (5). Those clade Ib–driven outbreaks nonetheless include severe cases, underscoring the need to elucidate genetic determinants of virulence within and between MPXV lineages.
During December 2023–August 2024, we collected 41 clinical samples (lesion swabs, crusts, whole blood, and serum) from 21 persons in Liberia suspected to have mpox. We recorded epidemiologic and clinical data on a standardized form based on the Integrated Disease Surveillance and Response Technical Guidelines (https://www.who.int/publications/i/item/WHO-AF-WHE-CPI-05-2019#:~:text=The%20third%20edition%20of%20the%20Integrated%20Disease%20Surveillance,and%20the%20U.S.%20Agency%20for%20International%20Development%20%28USAID%29). In August 2024, an mpox outbreak caused by clade Ib MPXV was declared a public health emergency of international concern (6), whereas clade IIb continued to circulate globally. Diagnostic testing at the National Public Health Institute of Liberia National Reference Laboratory (https://nphil.gov.lr) used an MPXV real-time PCR (Liferiver Bio-Tech Corp., http://www.liferiverbiotech.com) for initial virus detection and confirmation (Table) (7).
To determine which MPXV clades were circulating in Liberia, the National Public Health Institute of Liberia partnered with the Integrated Research Facility at Fort Detrick (IRF-Frederick) to perform genomic sequencing. That collaboration aimed to identify previously undetected MPXV strains and possible co-infections to inform public health measures and clinical management.
We transferred 41 inactivated specimens from 21 patients to IRF-Frederick, which extracted nucleic acids by using a MagMAX Viral/Pathogen Nucleic Acid Isolation Kit (Thermo Fisher Scientific, https://www.thermofisher.com) on a KingFisher Flex system (Thermo Fisher Scientific). We prepared sequencing libraries by using the DNA Prep with Enrichment kit with 10- to 49-ng input (Illumina, https://www.illumina.com) and enriched the libraries by using the Comprehensive Viral Research Panel (Twist Biosciences, https://www.twistbioscience.com). We prepared the libraries by using unique dual indices (IDT for Illumina UDIs, Set A; Illumina) followed by precapture pooling assembled in 6-plex with up to 500 ng per library. After capture, we pooled all libraries equimolarly and loaded the libraries on a single NextSeq 1000/2000 P1 XLEAP-SBS 300-cycle flow cell (2 × 150 bp; Illumina). This enrichment-based metagenomic sequencing approach enabled detection of MPXV and other viral DNA pathogens present in the samples.
We analyzed sequencing reads with EsViritu version 0.2.3 (https://github.com/cmmr/EsViritu) to identify viruses and quantify viral genomes. We further processed MPXV reads by using the nf-core/viralrecon pipeline version 2.6.0 (https://nf-co.re/viralrecon/2.6.0/) and aligned the reads to a reference MPXV strain rom GenBank (accession no. KJ642613.1).
Overall, we detected MPXV DNA in 10 patients and varicella zoster virus (VZV) in 10 patients, including 2 cases of possible MPXV–VZV co-infection (Appendix Figure 1). We also detected partial genomes of Epstein-Barr virus (Herpesviridae: Lymphocryptovirus humangamma 4), hepatitis B virus (Hepadnaviridae: Orthohepadnavirus hominoidei), and torque teno mini virus 8 (Anelloviridae: Betatorquevirus homini 8). Detection of multiple viral pathogens, including VZV and MPXV within the same time frame, reinforces the value of broad metagenomic approaches for diagnosing lesions of unknown etiology, particularly when clinical manifestations overlap.
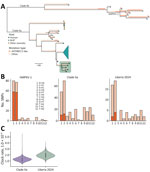
We assembled 5 near-complete (>98%) MPXV genomes; Nextclade (https://docs.nextstrain.org/projects/nextclade/en/stable/index.html) analysis confirmed that all belonged to clade IIa. We deposited all sequences in GenBank (accession nos. PV122071–5). To determine whether those MPXV cases arose from zoonotic spillover or ongoing human-to-human transmission, we analyzed phylogenetic relationships, mutation rates, and apolipoprotein B mRNA editing enzyme, catalytic subunit 3G (APOBEC3)–mediated editing patterns (Figure; Appendix Figure 2). Maximum-likelihood analysis and APOBEC3 ancestral reconstruction using squirrel version 1.0.11 (https://github.com/aineniamh/squirrel) showed limited APOBEC3-mediated editing (6/61 internal single-nucleotide polymorphisms [9.8%]). By contrast, clade IIb viruses from the 2022 global outbreak exhibited extensive APOBEC3-driven hypermutation (8).
Next, by using a fixed local clock model in BEAST version 1.10.5 (https://beast.community), we estimated a mean evolutionary rate of 1.96 × 10−6 substitutions/site/year (95% highest posterior probability 7.61 × 10−7 to 3.92 × 10−6) for the clade IIa sequences. That rate is ≈15-fold lower than the APOBEC3-driven rates reported for 2022 clade IIb strains (9), further indicating that those infections reflect spillover events rather than sustained human-to-human transmission.
Finally, because MPXV diversity is largely shaped by geographic separation rather than time (10), the distinct phylogenetic clustering of cases from Sinoe County versus those from Nimba County (Appendix Figure 2) also supports independent zoonotic spillover events rather than a single transmission chain. However, inference is limited by the small number of available sequences.
In conclusion, this study contributes MPXV genomes from Liberia, addressing a multidecade absence of genomic data from human cases in western Africa. Continued monitoring and sequencing efforts are essential for identifying emerging virus lineages, informing targeted public health interventions, and guiding clinical management strategies to address the varied presentations associated with different MPXV clades.
Dr. Nyan is director-general of the National Public Health Institute of Liberia in 2024. He specializes in infectious disease diagnostics and innovation and biomedical and clinical research. Dr. Berry is employed by Laulima Government Solutions and serves as study director and the clinical studies support team lead at the National Institutes of Health, National Institute of Allergies and Infectious Diseases, Division of Clinical Research, Biosafety Level 4 facility, the integrated research facility at Fort Detrick, Frederick, Maryland, USA. Her research interests include evaluation of medical countermeasures, outbreak response and surveillance initiatives, and high-consequence and emerging or reemerging pathogens in high- and low-resource settings.
Acknowledgments
We thank Anya Crane for critically editing the manuscript and Jiro Wada for preparing figures. We also thank the Ministry of Health Liberia County Health Teams and National Public Health Institute of Liberia research and laboratory team members for their support in collection and transport of samples, as well as support with information about the mpox cases.
This study was approved under the public health sample collection policy of the Government of Liberia and received a nonhuman subject determination by the National Institutes of Health (NIH; IRF-Frederick project no. 2024-015), as defined by National Institutes of Health Policy Manual 3014-204. This work was supported in part through a Laulima Government Solutions, LLC, prime contract with the National Institute of Allergy and Infectious Diseases (NIAID), NIH (contract no. HHSN272201800013C). I.M.B., M.M., G.W., S.H., S.E.K., and S.E.Z. performed this work as employees of Laulima Government Solutions, LLC. J.H.K. performed this work as an employee of Tunnell Government Services, a subcontractor of Laulima Government Solutions, LLC (contract no. HHSN272201800013C). This work was also supported in part with federal funds from the National Cancer Institute (NCI), NIH (contract no. 75N910D00024, task order no. 75N91019F00130). I.C. was supported by the Clinical Monitoring Research Program Directorate, Frederick National Lab for Cancer Research, sponsored by NCI. This work was also supported in part with federal funds from an Oak Ridge Institute for Science and Education (ORISE) postdoctoral research fellowship program through and agreement with NIAID. S.E.Z. also performed this work as a postdoctoral fellow under the ORISE program.
References
- Happi C, Adetifa I, Mbala P, Njouom R, Nakoune E, Happi A, et al. Urgent need for a non-discriminatory and non-stigmatizing nomenclature for monkeypox virus. PLoS Biol. 2022;20:
e3001769 . DOIPubMedGoogle Scholar - Likos AM, Sammons SA, Olson VA, Frace AM, Li Y, Olsen-Rasmussen M, et al. A tale of two clades: monkeypox viruses. J Gen Virol. 2005;86:2661–72. DOIGoogle Scholar
- Masirika LM, Udahemuka JC, Schuele L, Ndishimye P, Otani S, Mbiribindi JB, et al. Ongoing mpox outbreak in Kamituga, South Kivu province, associated with monkeypox virus of a novel Clade I sub-lineage, Democratic Republic of the Congo, 2024. Euro Surveill. 2024;29:
2400106 . DOIGoogle Scholar - Mauldin MR, McCollum AM, Nakazawa YJ, Mandra A, Whitehouse ER, Davidson W, et al. Exportation of monkeypox virus from the African continent. J Infect Dis. 2022;225:1367–76. DOIGoogle Scholar
- Levy V, Branzuela A, Hsieh K, Getabecha S, Berumen R III, Saadeh K, et al. Clade I Mpox Response Team. First clade Ib monkeypox virus infection reported in the Americas–California, November 2024. MMWR Morb Mortal Wkly Rep. 2025;74:44–9. DOIGoogle Scholar
- World Health Organization. WHO director-general declares mpox outbreak a public health emergency of international concern [cited 2025 Jan 6]. https://www.who.int/news/item/14-08-2024-who-director-general-declares-mpox-outbreak-a-public-health-emergency-of-international-concern
- Schuele L, Masirika LM, Udahemuka JC, Siangoli FB, Mbiribindi JB, Ndishimye P, et al.; GREATLIFE MPOX group; Collaborators. Real-time PCR assay to detect the novel Clade Ib monkeypox virus, September 2023 to May 2024. Euro Surveill. 2024;29:
2400486 . DOIPubMedGoogle Scholar - Patrono LV, Pléh K, Samuni L, Ulrich M, Röthemeier C, Sachse A, et al. Monkeypox virus emergence in wild chimpanzees reveals distinct clinical outcomes and viral diversity. Nat Microbiol. 2020;5:955–65. DOIPubMedGoogle Scholar
- Gigante CM, Korber B, Seabolt MH, Wilkins K, Davidson W, Rao AK, et al. Multiple lineages of monkeypox virus detected in the United States, 2021–2022. Science. 2022;378:560–5. DOIGoogle Scholar
- Forni D, Molteni C, Cagliani R, Sironi M. Geographic structuring and divergence time frame of monkeypox virus in the endemic region. J Infect Dis. 2023;227:742–51. DOIGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: August 04, 2025
1These first authors contributed equally to this article.
Table of Contents – Volume 31, Number 9—September 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Sara E. Zufan, National Institutes of Health, 8200 Research Plaza, Fort Detrick, Frederick, MD 21702, USA
Top