Volume 30, Number 11—November 2024
Research Letter
Mpox Hepatic and Pulmonary Lesions in HIV/Hepatitis B Virus Co-Infected Patient, France
Cite This Article
Citation for Media
Abstract
We report a case of persistent disseminated mpox evolving over >6 months in an HIV/hepatitis B virus co-infected patient in France who had <200 CD4+ cells/mm3, pulmonary and hepatic necrotic lesions, persistent viremia, and nasopharyngeal excretion. Clinical outcome was favorable after 90 days of tecovirimat treatment and administration of human vaccinia immunoglobulins.
By November 2023, the global mpox outbreak that began in May 2022 had resulted in >92,000 cases and 171 deaths across 116 countries (1). Among HIV-infected persons, prevalence was high (27%–60%); the most severe and fatal outcomes were observed in those who had advanced infections (2,3). A new mpox strain in Africa prompted the World Health Organization to declare a global emergency (4). Despite ongoing clinical trials, no established guidelines exist for managing severe mpox cases (3). We report successful management of persistent disseminated mpox having nodular liver and bilateral lung involvement in an immunosuppressed patient co-infected with HIV and hepatitis B virus (HBV).
A 49-year-old HIV/HBV–co-infected patient who identified as a man who has sex with men was hospitalized in September 2022 for disseminated necrotizing mpox skin and anal lesions. Despite a low HIV-1 virus load (54 copies/mL) under multidrug therapy, his CD4+ cell count was low (82 cells/μL), and HBV virus load was high (8.02 log IU/mL) because of poor tenofovir adherence. A 14-day tecovirimat course (Appendix Figure) improved his skin and anal lesions. However, after discontinuing treatment, new cutaneous nodular lesions appeared, and existing lesions worsened. A computed tomography (CT) scan revealed a 4-cm necrotic mass in the right upper lung lobe, nodules in the opposite lung, and perirectal and nodular liver lesions. Rehospitalized and suspected of having metastatic cancer, he also had ulceronecrotic lesions on his foot, hands, and forearm.
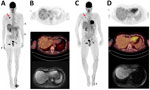
A positron emission tomography/CT scan showed hypermetabolic foci in the skin, rectum, lungs, and liver (Figure, panel A). Liver magnetic resonance imaging (MRI) (Figure, panel B) showed multiple 1–2 cm abscesses across all lobes. Brain MRI and cardiac ultrasound results were unremarkable. The foot lesion sample was positive for monkeypox virus (MPXV) by PCR (cycle threshold 13.47) (Appendix Figure). Anal and skin biopsies revealed massive necrosis; a liver biopsy showed necrotic tissue without tumor cells but had a high MPXV PCR result. Blood and nasopharyngeal swab samples tested positive for MPXV (Appendix Figure). A lung biopsy indicated the presence of pulmonary adenocarcinoma that was positive for MPXV but negative for other pathogens.
We readministered tecovirimat on November 25, 2022, and treated a secondary skin infection. After 1 month, the skin lesions deteriorated; blood and respiratory samples remained MPXV positive. Tecovirimat plasma levels were adequate, and virus sequencing at different time points revealed no resistance-associated mutations (i.e., F13L gene). We administered 2 doses of 6,000 IU/kg vaccinia immune globulin intravenous (VIGIV), which led to gradual improvement, although blood remained MPXV positive for 5 weeks. We continued tecovirimat treatment for 90 days.
We measured humoral responses to MPXV by using serologic and seroneutralization assays (Table) (5). Neutralizing antibody (NAb) titers in serum samples without added complement decreased when lesions reappeared but increased substantially after the first VIGIV injection. Nab titers in serum samples with added complement remained consistently high. Follow-up positron emission tomography/CT scan and MRI showed reduced lung and liver lesions (Figure, panels C, D). On February 7, 2023, we performed a right lobectomy and removed a 0.7-cm adenocarcinoma in a 2.5-cm necrotic mass; lymph nodes had no metastatic cells. By March 20, the hepatic lesions regressed, and the patient fully recovered, with no relapse as of November 2023.
MPXV can persist in HIV patients, causing prolonged lesions that might be fatal (6). However, disease persistence for >6 months is rare. Relapse after initial tecovirimat treatment is also uncommon; immune reconstitution inflammatory syndrome was considered, but it was unlikely because of the patient’s low virus load. A longer initial tecovirimat treatment course might have been beneficial (7,8). Disseminated MPXV with lung, gastrointestinal, and neurologic involvement in HIV patients has been documented (2,3,9), but liver nodules were unexpected. Although initial radiologic description suggested tumor lesions, biopsies confirmed MPXV was present without cancer cells. The lung adenocarcinoma, an incidental finding, was surgically managed, and the tumor tested positive for MPXV.
Tecovirimat effectiveness was limited, despite adequate plasma levels. Tecovirimat is generally considered safe, but its efficacy in treating mpox remains uncertain (3,10). Drug resistance was a concern because of the patient’s prolonged immunosuppression and MPXV replication, but virus sequencing revealed no resistance-associated mutations. Thus, we continued tecovirimat treatment for the maximum US Food and Drug Administration–approved duration of 90 days without adverse effects.
We administered VIGIV at day 31 of tecovirimat treatment, leading to gradual lesion improvement. Although lesions healed, blood was MPXV positive for 5 weeks. Nab titers (without complement) decreased before the second hospitalization, potentially reflecting clinical disease progression. The first VIGIV injection considerably increased NAb titers, but they quickly declined, suggesting that measuring Nab titers without adding complement to the serum sample might have more clinical relevance.
In conclusion, disseminated MPXV in HIV patients with low CD4 counts can cause prolonged, severe, and potentially fatal outcomes. This case highlights the need to monitor tecovirimat concentrations and resistance mutations and underscores the potential critical role of VIGIV treatment in severe mpox cases. As mpox continues to spread, atypical manifestations and severe forms need to be acknowledged and managed, especially in at-risk patients.
Dr. Calin is an infectious diseases specialist based in Paris, France. Her research interests focus on management of infectious complications in immunosuppressed patients, especially in the field of HIV.
Acknowledgment
The patient provided written informed consent.
References
- World Health Organization. Multi-country outbreak of mpox: external situation report 31. December 22, 2023 [cited 2024 May 23]. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20231222_mpox_external-sitrep_31.pdf
- Mitjà O, Alemany A, Marks M, Lezama Mora JI, Rodríguez-Aldama JC, Torres Silva MS, et al.; SHARE-NET writing group. Mpox in people with advanced HIV infection: a global case series. Lancet. 2023;401:939–49. DOIPubMedGoogle Scholar
- Shishido AA, Street S. Optimal management of severe mpox in patients with uncontrolled human immunodeficiency virus. J Med Virol. 2023;95:
e29277 . DOIPubMedGoogle Scholar - Harris E. As mpox cases surge in Africa, WHO declares a global emergency—here’s what to know. JAMA. 2024;332:862–4. DOIPubMedGoogle Scholar
- Hubert M, Guivel-Benhassine F, Bruel T, Porrot F, Planas D, Vanhomwegen J, et al. Complement-dependent mpox-virus-neutralizing antibodies in infected and vaccinated individuals. Cell Host Microbe. 2023;31:937–948.e4. DOIPubMedGoogle Scholar
- Triana-González S, Román-López C, Mauss S, Cano-Díaz AL, Mata-Marín JA, Pérez-Barragán E, et al. Risk factors for mortality and clinical presentation of monkeypox. AIDS. 2023;37:1979–85. DOIPubMedGoogle Scholar
- Martinez AE, Frattaroli P, Vu CA, Paniagua L, Mintz J, Bravo-Gonzalez A, et al. Successful outcome after treatment with cidofovir, vaccinia, and extended course of tecovirimat in a newly-diagnosed HIV patient with severe mpox: a case report. Vaccines (Basel). 2023;11:650. DOIPubMedGoogle Scholar
- Miller MJ, Cash-Goldwasser S, Marx GE, Schrodt CA, Kimball A, Padgett K, et al.; CDC Severe Monkeypox Investigations Team. Severe monkeypox in hospitalized patients—United States, August 10–October 10, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1412–7. DOIPubMedGoogle Scholar
- Simadibrata DM, Lesmana E, Pratama MIA, Annisa NG, Thenedi K, Simadibrata M. Gastrointestinal Symptoms of Monkeypox Infection: A systematic review and meta-analysis. J Med Virol. 2023;95:
e28709 . DOIPubMedGoogle Scholar - Grosenbach DW, Honeychurch K, Rose EA, Chinsangaram J, Frimm A, Maiti B, et al. Oral tecovirimat for the treatment of smallpox. N Engl J Med. 2018;379:44–53. DOIPubMedGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: October 20, 2024
Table of Contents – Volume 30, Number 11—November 2024
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Ruxandra Calin, Infectious Diseases Department, Tenon Hospital, AP-HP, Sorbonne University, INSERM 1135, 4 rue de la Chine 75020, Paris, France
Top