Volume 30, Number 12—December 2024
Research
Autochthonous Blastomyces dermatitidis, India
Cite This Article
Citation for Media
Abstract
Blastomyces spp. fungi, the causal agent of blastomycosis, are common in North America but do occur in other areas of the world. The most prevalent pathogen in the genus is B. dermatitidis. Most B. dermatitidis isolates originate from North America, but there are sporadic reports of B. dermatitidis recovery from Africa and Asia. High-quality reports that incorporate genetic information about the fungus outside North America have been rare. Genome sequencing of 3 fungal isolates from patients in India with chronic respiratory diseases revealed that the isolates belong to a genetically differentiated lineage of B. dermatitidis. Because the patients had no history of traveling outside of Asia, blastomycosis was most likely autochthonously acquired, which suggests a local population of B. dermatitidis. Our results suggest the endemic range of B. dermatitidis is larger than previously thought, calling for a reassessment of the geographic range of different agents of endemic mycoses.
Blastomycosis is a fungal disease prevalent among immunosuppressed patients (1–5) that can develop as a progressive disease with either pulmonary or extrapulmonary involvement (1–3,6–10). Blastomycosis is caused by Blastomyces spp., which are thermal dimorphic fungi with a saprophytic mycelial form in the soil and a pathogenic yeast form in hosts (2,3,6,11,12). The transition between those 2 forms is mediated by temperature; at ≈37°C, the mycelial (or conidia) form transforms into a yeast (13–15). Most often, human infections occur after breathing in fungal spores from the environment (16), but a very small fraction of cases have demonstrated sexual transmission from patient to patient (17,18).
B. dermatitidis is the most heavily studied species of Blastomyces (4,5,19–21) and is considered endemic to North America. Some reports have suggested the range of B. dermatitidis might extend outside North America (22,22–25), but the actual range of the species is debated (23,26). Reports of blastomycosis in Asia have been considered either of poor-quality diagnosis or imported (25,26). In addition, the identity of the causative agent of blastomycosis in those locales remains largely unknown. PCR-based assays suggest some of the isolates recovered from patients in India could be B. dermatitidis (27), but those reports predate the acknowledgment of the existence of multiple species of Blastomyces.
In this article, we report a cluster of cases of blastomycosis from India. By using genomewide sequencing and phylogenetic reconstruction, we determined the etiologic agent of blastomycosis in the 3 cases is B. dermatitidis. The B. dermatitidis isolates form a differentiated lineage from the isolates in North America. The 3 cases reported in this article have no patient history of travel outside of India, which suggest either secondary infections from a traveler (unlikely given the known mode of transmission of Blastomyces) or locally acquired infections.
Blastomyces-Like Isolates from India
Isolate Collection and Morphology
We obtained isolates from 3 patients who had chronic respiratory disease during 2006–2010 and were referred to the Medical Mycology laboratory, Vallabhbhai Patel Chest Institute, at the University of Delhi (Delhi, India) for identification of mycotic etiology. The 3 patients’ infections were unresponsive to antitubercular therapy. A detailed assessment of the patients’ travel history was taken; none reported any travel outside of India. No follow-up record is available for the patients.
We processed the samples by using standard protocols; they were anonymized before they were received. The isolates were cultured from lung aspirate, lymph node biopsy, and discharging sinus of the sternum (Appendix Table 1). We subcultured the isolates on Sabouraud dextrose agar (SDA) at 25°C to induce mycelial growth. To assess whether the isolates showed transition to a yeast form when exposed to 37°C, we inoculated conidia on pea seed agar and incubated the isolates for 7 days. We microscopically examined the isolates after mounting them with lactophenol cotton blue.
Genome Sequencing
Next, we used the cultures to extract DNA from each isolate as previously described (28,29). We pooled the resulting genomic libraries sequenced in a single S4 flow cell (2 × 150 bp read length) by using the NovaSeq 6000 (Illumina, https://www.illumina.com). The target sequencing depth was 40 (Appendix Table 2). This sequencing process was done to obtain whole genome sequences of each isolate, which enabled us to differentiate the isolates from India in comparison with isolates from North America. We have described all variant calling protocols, phylogenetic, and population genetic approaches in detail in the appendix (Appendix). We deposited all analytical code related to this study into GitHub (https://github.com/gjofre/BlastomycesIndia).
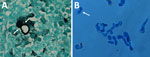
A histological section of 1 of the samples (subcutaneous, nodule sternum) revealed the presence of a large thick-walled broad-based budding yeast with a double refractile wall (Figure 1, panel A). This cell yeast morphology is typical of Blastomyces. The macroscopic mycelial colony morphologies were also consistent with that of Blastomyces. When grown on SDA at 25°C, all 3 isolates grew white downy colonies. We conducted microscopic examination of lactophenol cotton blue mounts from SDA slide cultures of each strain that revealed thin septate hyphae, bearing spherical to pyriform smooth-walled microconidia. Subcultured colonies on pea seed agar incubated at 37°C showed conversion from a mold to a yeast form. Finally, microscopic yeast morphology in culture was also consistent with that of Blastomyces. Similar to the yeast cells observed in the biopsy (Figure 1, panel A), microscopic examination of the growth of yeast revealed numerous thick-walled and broad-based budding yeast cells (Figure 1, panel B), which are typical of the genus (30).
Next, we obtained whole genome sequences for the 3 putative isolates of Blastomyces (mean coverage 20.47) (Appendix Table 1). A principal component analysis that included 2 genera from the dimorphic fungi strongly suggested the 3 isolates from India belonged to Blastomyces (Figure 2, panel A). Phylogenetic analyses confirmed that the 3 Blastomyces isolates from India are closely related to other isolates of B. dermatitidis and form a monophyletic group. This group is sister to the North America samples of B. dermatitidis. We recovered this topology regardless of the approach to generate the phylogenetic tree (Appendix Figure 1). In all cases, the branch is well supported (ultrafast bootstrap on 1,000 replicates = 100%). Assuming a mutation rate similar to that of other Ascomycetes (31,32), we found that the origin of the India clade of B. dermatitidis is not recent (divergence point estimate ≈0.8 million years, SE 0.12 million years) and might precede the settlement of modern humans in the Indian subcontinent (33,34).
Finally, we tested whether the monophyletic group of Blastomyces isolates from India were differentiated from the other isolates of B. dermatitidis. The India group showed the lowest level of diversity of any Blastomyces species, lower than that of the North American clade of B. dermatitidis and B. gilchristii (Table 2). The diversity values are similar in scale to the amount of variability in other fungal species (35). The extent of divergence between clades is much higher than π, a proxy of intraclade variability, for both the B. dermatitidis and B. gilchristii and for the B. dermatitis from India versus the rest of B. dermatitidis comparisons, suggesting genetic differentiation between the lineage from India and the other lineages of Blastomyces (Table 1) (36,37). Nonetheless, divergent statistics show there is a substantial level of admixture between B. dermatitidis from North America and India (Table 2; Appendix Table 3). Those results suggest that, despite the monophyly of this India Blastomyces group, the triad of isolates from India still exchanges genetic material with the North America lineage of B. dermatitidis.
In this article, we report a small cluster of cases of blastomycosis in patients from India with no travel history outside the country. By using genomewide sequencing, we determined that the 3 isolates are B. dermatitidis. To date, most cases of B. dermatitidis–caused blastomycosis outside of North America have had travel history to that continent (25). Because of the lack of evidence of person-to-person transmission in blastomycosis, the most likely explanation is that those cases were acquired from the environment. This hypothesis is consistent with environmental collections of B. dermatitidis from bat specimens in India (38,39).
Previous efforts have reported the occurrence of clinical cases of blastomycosis in India, and in all cases, the fungus was assumed to be B. dermatitidis; however, those reports preceded the description of other Blastomyces species and thus should be revisited. The first report of Blastomyces in a patient from India (40,41) revealed that 3 of 4 suspected cases had a positive immunodiffusion response against anti–B. dermatitidis serum precipitins. The efficacy of immunodiffusion to differentiate among Blastomyces species is unknown. Because the first report of Blastomyces in a patient from India predates DNA sequencing (40,41), it is unclear whether the samples included isolates from Blastomyces species other than B. dermatitidis. Previous reports from India have documented isolation of B. dermatitidis from insectivorous bats (38,39,41–43), and dogs (44), suggesting that the disease has an endemic niche in India. A second effort to characterize the global diversity of Blastomyces used restriction fragment-length polymorphisms to typify 52 isolates, including 3 isolates from India (45). That survey found 2 isolates from India (1 from a patient and 1 from a bat) were genetically similar to each other but were dissimilar from all other isolates included (45). Our phylogenetic reconstruction reveals a similar result in that the isolates from India reported in this article form a distinct genetic group within B. dermatitidis.
India has reported cases of blastomycosis imported from the United States (22,41). In those instances, the fungus was confirmed to be B. dermatitidis by DNA sequencing of 2 loci. Our phylogenetic analyses suggest that the lineage of B. dermatitidis we sampled in India diverged long ago from the North America lineage. Our results indicate that systematic collections of environmental and patient samples of Blastomyces, along with the use of genomics and phylogenetics, are needed to elucidate the natural occurrence and epidemiology of the etiologic agents of blastomycosis. Clinicians should be aware of the possibility of autochthonous blastomycosis in India, particularly among patients with chronic respiratory diseases.
Dr. Chowdhary is a medical mycologist and a physician scientist with the national reference laboratory for antimicrobial resistance in fungal pathogens. Her interests include human fungal infections and antimicrobial resistance.
Acknowledgments
We thank our reviewers and members of the Matute laboratory for helpful comments.
G.J.F., V.E.S., A.T.M., and D.R.M. were supported by the National Institute of Allergy and Infectious Disease of the National Institutes of Health (award no. R01AI153523). A.J.D. was supported under the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (award no. T32-AI052080).
References
- Castillo CG, Kauffman CA, Miceli MH. Blastomycosis. Infect Dis Clin North Am. 2016;30:247–64. DOIPubMedGoogle Scholar
- Klein BS, Vergeront JM, Davis JP. Epidemiologic aspects of blastomycosis, the enigmatic systemic mycosis. Semin Respir Infect. 1986;1:29–39.PubMedGoogle Scholar
- Mazi PB, Rauseo AM, Spec A. Blastomycosis. Infect Dis Clin North Am. 2021;35:515–30. DOIPubMedGoogle Scholar
- Schwartz IS, Wiederhold NP, Hanson KE, Patterson TF, Sigler L. Blastomyces helicus, a new dimorphic fungus causing fatal pulmonary and systemic disease in humans and animals in Western Canada and the United States. Clin Infect Dis. 2019;68:188–95. DOIPubMedGoogle Scholar
- Maphanga TG, Birkhead M, Muñoz JF, Allam M, Zulu TG, Cuomo CA, et al. Human blastomycosis in South Africa caused by Blastomyces percursus and Blastomyces emzantsi sp. nov., 1967–2014. J Clin Microbiol. 2020;58:e01661–19. DOIPubMedGoogle Scholar
- Gilchrist TC. A case of blastomycetic dermatitis in man. Johns Hopkins Hosp Rep. 1896;1(269–298):70.
- Gonyea EF. The spectrum of primary blastomycotic meningitis: a review of central nervous system blastomycosis. Ann Neurol. 1978;3:26–39. DOIPubMedGoogle Scholar
- Bariola JR, Perry P, Pappas PG, Proia L, Shealey W, Wright PW, et al. Blastomycosis of the central nervous system: a multicenter review of diagnosis and treatment in the modern era. Clin Infect Dis. 2010;50:797–804. DOIPubMedGoogle Scholar
- Martin DS, Smith DT. Blastomycosis, American blastomycosis, Gilchrist’s disease: I. A review of the literature. Am Rev Tuberc. 1939;39:275–304.
- Martin DS, Smith DT. Blastomycosis, American blastomycosis, Gilchrist’s disease: II. A report of thirteen new cases. Am Rev Tuberc. 1939;39:488–515.
- Klein BS, Vergeront JM, Weeks RJ, Kumar UN, Mathai G, Varkey B, et al. Isolation of Blastomyces dermatitidis in soil associated with a large outbreak of blastomycosis in Wisconsin. N Engl J Med. 1986;314:529–34. DOIPubMedGoogle Scholar
- Gilchrist TC, Stokes WR. A case of pseudo-lupus vulgaris caused by a Blastomyces. J Exp Med. 1898;3:53–78. DOIPubMedGoogle Scholar
- Fierer J. Invasive endemic fungi of the Western Hemisphere. Virulence. 2019;10:832–4. DOIPubMedGoogle Scholar
- Levine S, Ordal ZJ. Factors influencing the morphology of Blastomyces dermatitidis. J Bacteriol. 1946;52:687–94. DOIPubMedGoogle Scholar
- McBride JA, Gauthier GM, Klein BS. Turning on virulence: mechanisms that underpin the morphologic transition and pathogenicity of Blastomyces. Virulence. 2019;10:801–9. DOIPubMedGoogle Scholar
- Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367–81. DOIPubMedGoogle Scholar
- Farber ER, Leahy MS, Meadows TR. Endometrial blastomycosis acquired by sexual contact. Obstet Gynecol. 1968;32:195–9.PubMedGoogle Scholar
- Craig MW, Davey WN, Green RA. Conjugal blastomycosis. Am Rev Respir Dis. 1970;102:86–90.PubMedGoogle Scholar
- Jiang Y, Dukik K, Muñoz JF, Sigler L, Schwartz IS, Govender NP, et al. Phylogeny, ecology and taxonomy of systemic pathogens and their relatives in Ajellomycetaceae (Onygenales): Blastomyces, Emergomyces, Emmonsia, Emmonsiellopsis. Fungal Divers. 2018;90:245–91. DOIGoogle Scholar
- Brown EM, McTaggart LR, Zhang SX, Low DE, Stevens DA, Richardson SE. Phylogenetic analysis reveals a cryptic species Blastomyces gilchristii, sp. nov. within the human pathogenic fungus Blastomyces dermatitidis. PLoS One. 2013;8:
e59237 . DOIPubMedGoogle Scholar - Schwartz IS, Wiederhold NP, Patterson TF, Sigler L. Blastomyces helicus, an emerging dimorphic fungal pathogen causing fatal pulmonary and disseminated disease in humans and animals in western Canada and United States. Open Forum Infect Dis. 2017;4(Suppl 1):S83–S84. DOIGoogle Scholar
- Kumar A, Kunoor A, Eapen M, Singh PK, Chowdhary A. Blastomycosis misdiagnosed as tuberculosis, India. Emerg Infect Dis. 2019;25:1776–7. DOIPubMedGoogle Scholar
- Chakrabarti A, Slavin MA. Endemic fungal infections in the Asia-Pacific region. Med Mycol. 2011;49:337–44. DOIPubMedGoogle Scholar
- Salzer HJ, Stoney RJ, Angelo KM, Rolling T, Grobusch MP, Libman M, et al. Epidemiological aspects of travel-related systemic endemic mycoses: a GeoSentinel analysis, 1997–2017. J Travel Med. 2018;25(1):. DOIPubMedGoogle Scholar
- Gugnani HC, Sharma A, Sood N. A review of blastomycosis in Indian subcontinent. Eur J Med Health Sci. 2022;4:01–7.
- Ashraf N, Kubat RC, Poplin V, Adenis AA, Denning DW, Wright L, et al. Re-drawing the maps for endemic mycoses. Mycopathologia. 2020;185:843–65. DOIPubMedGoogle Scholar
- Yates-Siilata KE, Sander DM, Keath EJ. Genetic diversity in clinical isolates of the dimorphic fungus Blastomyces dermatitidis detected by a PCR-based random amplified polymorphic DNA assay. J Clin Microbiol. 1995;33:2171–5. DOIPubMedGoogle Scholar
- Jofre GI, Singh A, Mavengere H, Sundar G, D’Agostino E, Chowdhary A, et al. An Indian lineage of Histoplasma with strong signatures of differentiation and selection. Fungal Genet Biol. 2022;158:
103654 . DOIPubMedGoogle Scholar - Sepúlveda VE, Márquez R, Turissini DA, Goldman WE, Matute DR. Genome sequences reveal cryptic speciation in the human pathogen Histoplasma capsulatum. MBio. 2017;8:e01339–17. DOIPubMedGoogle Scholar
- Linder KA, Kauffman CA, Miceli MH. Blastomycosis: a review of mycological and clinical aspects. J Fungi (Basel). 2023;9:117. DOIPubMedGoogle Scholar
- Lynch M, Sung W, Morris K, Coffey N, Landry CR, Dopman EB, et al. A genome-wide view of the spectrum of spontaneous mutations in yeast. Proc Natl Acad Sci U S A. 2008;105:9272–7. DOIPubMedGoogle Scholar
- Farlow A, Long H, Arnoux S, Sung W, Doak TG, Nordborg M, et al. The spontaneous mutation rate in the fission yeast Schizosaccharomyces pombe. Genetics. 2015;201:737–44. DOIPubMedGoogle Scholar
- Thangaraj K, Chaubey G, Kivisild T, Reddy AG, Singh VK, Rasalkar AA, et al. Reconstructing the origin of Andaman Islanders. Science. 2005;308:996–996. DOIPubMedGoogle Scholar
- Tamang R, Thangaraj K. Genomic view on the peopling of India. Investig Genet. 2012;3:20. DOIPubMedGoogle Scholar
- Leffler EM, Bullaughey K, Matute DR, Meyer WK, Ségurel L, Venkat A, et al. Revisiting an old riddle: what determines genetic diversity levels within species? PLoS Biol. 2012;10:
e1001388 . DOIPubMedGoogle Scholar - Matute DR, Sepúlveda VE. Fungal species boundaries in the genomics era. Fungal Genet Biol. 2019;131:
103249 . DOIPubMedGoogle Scholar - Cai L, Giraud T, Zhang N, Begerow D, Cai G, Shivas RG. The evolution of species concepts and species recognition criteria in plant pathogenic fungi. Fungal Divers. 2011;50:121–33. DOIGoogle Scholar
- Khan ZU, Randhawa HS, Lulla M. Isolation of Blastomyces dermatitidis from the lungs of a bat, Rhinopoma hardwickei hardwickei Gray, in Delhi. Sabouraudia. 1982;20:137–44. DOIPubMedGoogle Scholar
- Randhawa HS, Chaturvedi VP, Kini S, Khan ZU. Blastomyces dermatitidis in bats: first report of its isolation from the liver of Rhinopoma hardwickei hardwickei Gray. Sabouraudia. 1985;23:69–76. DOIPubMedGoogle Scholar
- Randhawa HS, Khan ZU, Gaur SN. Blastomyces dermatitidis in India: first report of its isolation from clinical material. Sabouraudia. 1983;21:215–21. DOIPubMedGoogle Scholar
- Randhawa HS, Chowdhary A, Kathuria S, Roy P, Misra DS, Jain S, et al. Blastomycosis in India: report of an imported case and current status. Med Mycol. 2013;51:185–92. DOIPubMedGoogle Scholar
- Chaturvedi VP, Randhawa HS, Kini S, Khan ZU. Survival of Blastomyces dermatitidis in the gastrointestinal tract of an orally infected insectivorous bat, Rhinopoma hardwickei hardwickei Gray. J Med Vet Mycol. 1986;24:349–52. DOIPubMedGoogle Scholar
- Raymond JT, White MR, Kilbane TP, Janovitz EB. Pulmonary blastomycosis in an Indian fruit bat (Pteropus giganteus). J Vet Diagn Invest. 1997;9:85–7. DOIPubMedGoogle Scholar
- Sestero CM, Scalarone GM. Detection of IgG and IgM in sera from canines with blastomycosis using eight Blastomyces dermatitidis yeast phase lysate antigens. Mycopathologia. 2006;162:33–7. DOIPubMedGoogle Scholar
- McCullough MJ, DiSalvo AF, Clemons KV, Park P, Stevens DA. Molecular epidemiology of Blastomyces dermatitidis. Clin Infect Dis. 2000;30:328–35. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: November 22, 2024
Table of Contents – Volume 30, Number 12—December 2024
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Daniel R. Matute, Department of Biology, University of North Carolina, 3161 Genome Sciences building, CB 3280, Chapel Hill, NC 27599, USA
Top