Volume 30, Number 4—April 2024
Research Letter
Detection of Rat Hepatitis E Virus in Pigs, Spain, 2023
Abstract
We identified rat hepatitis E virus (HEV) RNA in farmed pigs from Spain. Our results indicate that pigs might be susceptible to rat HEV and could serve as viral intermediaries between rodents and humans. Europe should evaluate the prevalence of rat HEV in farmed pigs to assess the risk to public health.
Hepatitis E virus (HEV) is a major cause of acute viral hepatitis in Europe. HEV is classified into 8 major genotypes, but zoonotic genotype 3 is the most prevalent on the continent (1). HEV was considered the only zoonotic species in the Hepeviridae family until rat HEV (Rocahepevirus ratti) was identified. Rat HEV was the causal agent of chronic hepatitis in a transplant recipient from Hong Kong in 2018 (2). Since that discovery, nearly 30 cases of chronic and acute hepatitis have been reported in America, Asia, and Europe (3–6), affecting both immunosuppressed and immunocompetent persons. Those cases highlight the zoonotic potential of rat HEV, emphasizing its growing concern to public health.
Rodents are the main host of rat HEV, but its transmission routes remain unclear. Although direct and indirect contact with rodents have been suggested as potential transmission routes, only 1 registered case has involved such contact (6). Thus, alternative sources of infection seem possible, potentially from an alternate host with which humans have more contact (7). Because domestic pigs (Sus scrofa domestica) are highly susceptible to HEV and constitute the main natural viral reservoir, they could also be susceptible to rat HEV and potentially serve as hosts. Confirming that hypothesis could have major implications for public health. We aimed to assess the presence of rat HEV in a population of farmed pigs in Spain.
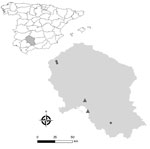
During May–June 2023, we randomly selected and prospectively sampled domestic pigs from 5 intensive breeding system farms in Cordoba, southern Spain. We collected rectal fecal samples from each pig and stored samples at −80°C until RNA extraction (Appendix).
We included a total of 387 pigs in the study and found rat HEV in 44 pigs, an individual prevalence of 11.4% (95% CI 8.6%–14.9%) (Table). Sequencing confirmed the identity as rat HEV (species R. ratti) (GenBank accession nos. OR977681–7 and OR977689–7711) (Appendix Figures 1, 2). Among the 5 farms, 2 (40%) had >1 rat HEV–positive pig. Of note, 93.2% (41/44) of positive animals were from the same farm (Figure; Appendix Table 3). HEV RNA was detected in 6 pigs, indicating a prevalence of 1.6% (95% CI 0.6%–3.4%). All HEV-positive pigs were from the same farm and had sequences consistent with HEV genotype 3f (GenBank accession nos. OR818554–60), but rat HEV was not detected in that farm.
The hypothesis that pigs are not susceptible to rat HEV was formed on the basis of experimental in vivo studies (8). Because animals in that study were not infected after challenge with rat HEV strains (8), it appeared that pigs were resistant to rat HEV infection. However, our study detected rat HEV RNA in pigs, suggesting the possible role of pigs in rat HEV epidemiology. That finding increases the range of species susceptible to rat HEV, suggesting that its transmission might not be restricted to rodents. The number of positive animals we found suggests that rat HEV is widespread among pig populations in the study area. That observation might be linked to the elevated positivity rate (55%) discovered in rodents from the same region (9), implying that the lack of rodent control measures might increase the risk for rat HEV transmission.
The presence of rat HEV in farmed pigs is of public health concern, especially considering global pork consumption. Our study highlights the possibility that pigs intended for human consumption could contribute to rat HEV transmission. The European Food Safety Authority (EFSA) recommends monitoring HEV in pigs to identify alterations in virus distribution and prevent its spread to new farms, aiming to reduce human cases (10). Our results suggest that a preliminary evaluation of rat HEV in farmed pigs should be also conducted in Europe, which could confirm our results and increase our understanding of virus transmission.
The first limitation of our study is that because of its exploratory nature, the sampling area was restricted to a single region, but our findings underscore the need to extend the evaluation of rat HEV to determine its magnitude. Second, because no serologic assays are available for detecting rat HEV antibodies in pigs, our analysis was limited to molecular testing on fecal samples; thus, we cannot confirm rat HEV infection. However, our study justifies the design of new studies to evaluate the presence of rat HEV in blood and tissues samples. Finally, implementation of serologic analysis on rat HEV might enhance our comprehension of the pathogenesis of both HEV and rat HEV and assist in future investigations into risk factors.
In conclusion, our study shows the possibility that pigs are susceptible to rat HEV infection, challenging previous assumptions. Further studies are warranted to determine the role of pigs in rat HEV epidemiology and to assess the risk for direct or indirect zoonotic transmission from pigs. In addition, Europe should conduct an evaluation of rat HEV in farmed pigs to assess the overall risk to public health.
Ms. Ríos-Muñoz is a researcher specializing in infectious zoonotic diseases and a PhD candidate at Universidad de Córdoba, Córdoba, Spain. Her research interests focus on hepatitis E virus and rat hepatitis E virus.
Acknowledgments
We gratefully acknowledge Laura Ruiz Torres and Ismael Zafra Soto for their technical support in sample processing and analysis.
This work was supported by the Andalusian General Secretariat for Research, Development, and Innovation in Health (grant no. PI-0287-2019), the Spanish Ministry of Health (grant no. RD12/0017/0012), co-financed by European Regional Development Fund (ERDF), and the Carlos III Health Institute (grant nos. PI21/00793 and PI22/01098). Projects PI21/00793 and PI22/01098 were funded by Carlos III Health Institute (ISCIII) and co-funded by the European Union.
A.R.-J. is the recipient of a Miguel Servet Research Contract by the Spanish Ministry of Sciences (contract no. CP18/00111). J.C.-G. is supported by the CIBERINFEC (grant no. CB21/13/00083), Carlos III Health Institute, Spanish Ministry of Science and NextGenerationEU. L.R.-M. is the recipient of a INVESTIGO research program grant funded by the European Union NextGenerationEU Plan. M.C.-J. is the recipient of a PFIS predoctoral grant (grant no. FI22/00180) from the Carlos III Health Institute and co-funded by the European Union. D.C.-M. is the recipient of a Rio-Hortega grant (grant no. CM22/00176) from the Carlos III Health Institute and co-funded by the European Union. M.G. was supported by postdoctoral contract Margarita Salas from the University of Murcia, and P.L.-L. was supported by postdoctoral contract Margarita Salas from the University of Córdoba from the Program of Requalification of the Spanish University System (Spanish Ministry of Universities) financed by the European Union-NextGenerationEU. S.C.-S. and I.A.-R. hold an FPU grants from the Spanish Ministry of Universities (grant no. FPU19/06026 to S.C.-S. and grant no. FPU19/03969 to I.A.-R.). The funders did not play any role in the design, conclusions, interpretation of the study, or decision to publish.
Author contributions: L.R.-M., A.R.-J., and A.R. were involved in the study design and conception, interpretation of the data, drafting of the manuscript, study supervision, and funding obtention. M.G., S.C.-S., T.F., and I.A.-R. were involved in sampling design, collection and storage. L.R.-M., P.L.-L., M.C.-J., and J.C.-G. performed RNA extraction and molecular determinations, phylogenetic analysis and GenBank submission. All authors have revised the manuscript and approved its publication.
References
- Adlhoch C, Avellon A, Baylis SA, Ciccaglione AR, Couturier E, de Sousa R, et al. Hepatitis E virus: Assessment of the epidemiological situation in humans in Europe, 2014/15. J Clin Virol. 2016;82:9–16. DOIPubMedGoogle Scholar
- Sridhar S, Yip CCY, Wu S, Cai J, Zhang AJX, Leung KH, et al. Rat hepatitis E virus as cause of persistent hepatitis after liver transplant. Emerg Infect Dis. 2018;24:2241–50. DOIPubMedGoogle Scholar
- Rivero-Juarez A, Frias M, Perez AB, Pineda JA, Reina G, Fuentes-Lopez A, et al.; HEPAVIR and GEHEP-014 Study Groups. Orthohepevirus C infection as an emerging cause of acute hepatitis in Spain: First report in Europe. J Hepatol. 2022;77:326–31. DOIPubMedGoogle Scholar
- Rodriguez C, Marchand S, Sessa A, Cappy P, Pawlotsky JM. Orthohepevirus C hepatitis, an underdiagnosed disease? J Hepatol. 2023;79:e39–41. DOIPubMedGoogle Scholar
- Andonov A, Robbins M, Borlang J, Cao J, Hatchette T, Stueck A, et al. Rat hepatitis E virus linked to severe acute hepatitis in an immunocompetent patient. J Infect Dis. 2019;220:951–5. DOIPubMedGoogle Scholar
- Sridhar S, Yip CCY, Lo KHY, Wu S, Situ J, Chew NFS, et al. Hepatitis E virus species C infection in humans, Hong Kong. Clin Infect Dis. 2022;75:288–96. DOIPubMedGoogle Scholar
- Reuter G, Boros Á, Pankovics P. Review of hepatitis E virus in rats: evident risk of species Orthohepevirus C to human zoonotic infection and disease. Viruses. 2020;12:1148. DOIPubMedGoogle Scholar
- Cossaboom CM, Córdoba L, Sanford BJ, Piñeyro P, Kenney SP, Dryman BA, et al. Cross-species infection of pigs with a novel rabbit, but not rat, strain of hepatitis E virus isolated in the United States. J Gen Virol. 2012;93:1687–95. DOIPubMedGoogle Scholar
- Casares-Jimenez M, Garcia-Garcia T, Suárez-Cárdenas JM, Perez-Jimenez AB, Martín MA, Caballero-Gómez J, et al. Correlation of hepatitis E and rat hepatitis E viruses urban wastewater monitoring and clinical cases. Sci Total Environ. 2024;908:
168203 . DOIPubMedGoogle Scholar - Nigsch A. Surveillance plan proposal for early detection of zoonotic pathogens in pigs and poultry. EFSA Supporting Publications. 2023;20:1–32. DOIGoogle Scholar
Figure
Table
Cite This ArticleOriginal Publication Date: March 18, 2024
Table of Contents – Volume 30, Number 4—April 2024
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Antonio Rivero-Juarez, Virología Clínica y Zoonosis, Instituto Maimonides de Investigación Biomédica de Córdoba (IMIBIC), Avenida Menedez Pidal, s/n. 14004, Córdoba, Spain
Top