Volume 30, Number 9—September 2024
Dispatch
Avian and Human Influenza A Virus Receptors in Bovine Mammary Gland
Cite This Article
Citation for Media
Abstract
An outbreak of influenza A (H5N1) virus was detected in dairy cows in the United States. We detected influenza A virus sialic acid -α2,3/α2,6-galactose host receptors in bovine mammary glands by lectin histochemistry. Our results provide a rationale for the high levels of H5N1 virus in milk from infected cows.
Influenza A virus (IAV) is a negative, single-stranded RNA virus. Viral evolution has enabled some IAVs to cross species barriers and to be established in humans and various mammals (1). Cattle are susceptible to infection with influenza C and D viruses but have been regarded as almost resistant to infection with IAV (2). An unexpected highly pathogenic avian influenza (HPAI) virus H5N1 (clade 2.3.4.4b) was detected in dairy cattle in Texas, USA, and has spread to 131 herds in 12 different states in the United States (3,4). Although extremely high levels of virus in milk from infected cows (5) were unexpected, previous studies and a report from Friedrich-Loeffler Institute (FLI; Insel Riems, Germany) (6) have shown that the inoculation of IAVs into the mammary glands of cows and goats results in productive viral infection (2).
Hemagglutinin (HA) binds to sialic acids (SA) terminally attached to glycans, enabling viral endocytosis and membrane fusion. Hemagglutinins of human- and swine-adapted IAVs frequently prefer SAs linked to galactose (Gal) in an α2,6 linkage (SA-α2,6, human receptor), whereas avian IAVs prefer an α2,3 linkage (SA-α2,3, avian receptor) (7). Furthermore, IAVs adapted to chickens generally prefer SA-α2,3-Gal with a β1,4 linkage to N-acetylgalactosamine (GalNac; SA-α2,3-Gal-β1,4-GalNac, referred to as chicken receptor), whereas IAVs isolated from ducks favor SA-α2,3-Gal with a β1,3 linkage to N-acetylglucosamine (GlcNac; SA-α2,3-Gal-β1,3-GlcNac, referred to as duck receptor) (7).
To investigate IAV receptor expression on the surface of epithelial cells, in situ techniques, such as lectin histochemistry, are useful (8). A limitation of using lectins is that they provide information only about the terminal end of the host receptors; a complete quantification of the receptors is not possible. Three studies have reported the IAV receptors in the bovine respiratory tract by using lectins (9–11), but studies describing the IAV receptor distribution in other bovine tissues are sparse. The aim of this study was to investigate the in situ expression of IAV receptors in the bovine mammary glands by lectin histochemistry.
We included 2 archived bovine mammary glands obtained from the same lactating dairy cow (4 years of age) of a Danish Holstein breed and included mammary glands from 8 cows of different breeds and ages obtained from a slaughterhouse in Denmark (Table). The tissues were formalin-fixed, paraffin-embedded, and cut into 4–5 µm sections.
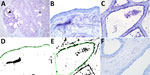
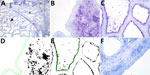
We detected the human receptor SA-α2,6 using biotinylated Sambucus nigra lectin (SNA) (B-1305-2; Vector Laboratories, https://vectorlabs.com). We detected the chicken receptor SA-α2,3-Gal-β1,4 using biotinylated Maackia amurensis lectin I (MAA-I) (B-1315-2; Vector Laboratories) and the duck receptor (SA-α2,3-Gal-β1,3) biotinylated MAA-II (B-1265-1; Vector Laboratories) as previously described (13). We semiquantified the staining on the surface of the epithelial cells as the percentage of positive surface area in 2 images from each slide (Figure 1, panels D, E; Figure 2, panels D, E) and reported the average (13). We applied 2 negative controls. To investigate potential background staining (blind), we added no lectins; to investigate the amount of nonspecific binding, we applied a neuraminidase pretreatment before each lectin, as previously reported (13). We included a confirmed IAV-negative pig lung as a positive control.
In the mammary gland, the human receptor (detected by SNA) and the duck receptor (detected by MAA-II) were widely distributed in the alveoli, but less so in the ducts, whereas we detected no positive staining of the chicken receptor by MAA-I (Table; Figure 1; Figure 2; Appendix Tables), except for 1 cow expressing the chicken receptor in a lactiferous duct. Both human and duck receptors were primarily expressed in the active alveoli and less expressed in less active alveoli (Figure 1; Figure 2). We found a significant negative correlation between the percentage of SNA staining in the alveoli and the parity status of the cows but not with the MAA-II lectin staining (Figure 3).
We detected nonspecific staining (positive staining after neuraminidase pretreatment) in the endothelial cells with the SNA and MAA-I lectins, in the bovine erythrocytes with the MAA-I lectin, and in the ducts of the MAA-II lectin, but observed no nonspecific staining elsewhere (Appendix Figure). The positive controls of the pig lung corresponded to previous findings (13) (Appendix Figure).
Our investigation evaluated the expression of IAV receptors in situ in the mammary gland of cattle, which typically has been considered less susceptible to IAV infection (2). Of note was the finding that both the human receptors (SA-α2,6) and the duck receptors (SA-α2,3-Gal-β1,3) were highly expressed in the active alveoli in mammary glands but lower expressed in the less active alveoli, which could indicate that cows peaking in lactation are more susceptible for infection. The findings of the 2 receptors are in agreement with 2 novel studies investigating the IAV receptors in the bovine mammary gland of 2–3 cows (11; M.R. Carrasco et al., unpub. data, https://doi.org/10.1101/2024.05.24.595667). The transmission routes and the pathogenesis of H5N1 in cows remain unclear, but the virus remains infectious after 1 hour on the milking equipment (V.L. Sage et al., unpub. data, https://doi.org/10.1101/2024.05.22.24307745); the US Department of Agriculture has reported that only some udder quarters may be involved in infection, suggesting an ascending infection as a possible transmission route. Of interest, the human and duck receptors were less expressed in the ducts of the mammary gland, making an ascending mammary gland infection using those receptors more challenging. An investigation of the receptor binding preference of A/Texas/37/2024 showed that an I199T mutation of the hemagglutinin protein increased the receptor binding breadth to a higher number of conformations of the avian receptor, including hybrid N-glycans, compared with previous H5N1 viruses (M.R. Good et al., unpub. data, https://doi.org/10.1101/2024.06.22.600211); those hybrid N-glycans might not be detected by the lectins used in our study (14). M.R. Carrasco, et al. (unpub. data) showed limited binding of older AIV strains and a mouse-adapted human influenza strain (PR8) in the mammary gland of cows, whereas the preliminary report from FLI (6) showed that genotypes from Europe of the clade 2.3.4.4b H5 HPAI viruses could also replicate in the mammary glands of cows. Thus, more studies are needed to investigate the susceptibility of cows to different IAV strains and variants.
The SNA lectin detects both of the 2 most common sialic acids, N-acetylneuraminic acid (Neu5Ac) and N-glycolylneuraminic acid (Neu5Gc) (14). Neu5Ac is mainly the preferred SA for IAVs, except for equine IAVs, which have a higher preference for Neu5Gc (7). Cattle express both Neu5Ac and Neu5Gc in their tissues, but the amount of Neu5Ac in bovine milk is 30 times higher than for Neu5Gc (15), which indicates that the SNA staining detected in our study was caused by staining of Neu5Ac. However, further studies such as mass spectrometry, which gives detailed information about the IAV receptors (e.g., sialic acid type, length, branching), are needed to confirm the cause of SNA staining and also to accomplish a comprehensive quantification of the distribution of receptors in bovine tissues (8).
The expression of the duck receptor in the mammary gland of cows fits with the observed widespread infections among cattle in the United States with HPAI H5N1. The co-expression of both human and avian receptors in the mammary glands indicates susceptibility to other IAVs than those from avian origin, which is worrying from a zoonotic perspective. The presence of the IAV receptors, however, does not provide evidence that cattle are susceptible to all IAVs; other host factors (1) probably play a role for successful replication.
Dr. Kristensen is a postdoctoral researcher at the University of Copenhagen in the virology and pathology research fields. Her primary focus is zoonotic influenza A viruses.
Acknowledgment
This article was preprinted at https://www.biorxiv.org/content/10.1101/2024.05.03.592326v1.
References
- Yoon S-W, Webby RJ, Webster RG. Evolution and ecology of influenza A viruses. In: Compans R, Oldstone M, editors. Influenza pathogenesis and control. Vol. I. Current topics in microbiology and immunology. Cham (Switzerland); Springer; 2014. p 359–75.
- Sreenivasan CC, Thomas M, Kaushik RS, Wang D, Li F. Influenza A in bovine species: a narrative literature review. Viruses. 2019;11:561. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. H5N1 bird flu: current situation summary. [cited 2024 Jun 28]. https://www.cdc.gov/flu/avianflu/avian-flu-summary.htm
- Ly H. Highly pathogenic avian influenza H5N1 virus infections of dairy cattle and livestock handlers in the United States of America. Virulence. 2024;15:
2343931 . DOIPubMedGoogle Scholar - World Health Organization. Joint FAO/WHO/WOAH preliminary assessment of recent influenza A(H5N1) viruses. 23 April 2024 [cited 2024 Jun 24]. https://www.who.int/publications/m/item/joint-fao-who-woah-preliminary-assessment-of-recent-influenza-a(h5n1)-viruses
- Friedrich-Loeffler-Institut. Rapid risk assessment for highly pathogenic avian influenza H5 (HPAI H5) clade 2.3.4.4b. 2024 [cited 2024 Jul 15]. https://www.openagrar.de/servlets/MCRFileNodeServlet/openagrar_derivate_00060240/FLI-Risikoeinschaetzung_HPAI_H5_2024-07-05_en.pdf
- Zhao C, Pu J. Influence of host sialic acid receptors structure on the host specificity of influenza viruses. Viruses. 2022;14:2141. DOIPubMedGoogle Scholar
- Varki NM, Varki A. Diversity in cell surface sialic acid presentations: implications for biology and disease. Lab Invest. 2007;87:851–7. DOIPubMedGoogle Scholar
- Thontiravong A, Rung-ruangkijkrai T, Kitikoon P, Oraveerakul K, Poovorawan Y. Influenza A virus receptor identification in the respiratory tract of quail, pig, cow and swamp buffalo. Wetchasan Sattawaphaet. 2011;41:15. DOIGoogle Scholar
- Uprety T, Sreenivasan CC, Bhattarai S, Wang D, Kaushik RS, Li F. Isolation and development of bovine primary respiratory cells as model to study influenza D virus infection. Virology. 2021;559:89–99. DOIPubMedGoogle Scholar
- Nelli RK, Harm TA, Siepker C, Groeltz-Thrush JM, Jones B, Twu NC, et al. Sialic acid receptor specificity in mammary gland of dairy cattle infected with highly pathogenic avian influenza A(H5N1) virus. Emerg Infect Dis. 2024;30:1361–73. DOIPubMedGoogle Scholar
- Hurley WL, Loor JJ. Mammary gland: growth, development and involution. In: Fuquay JW, Fox PF, McSweeney PLH, editors. Encyclopedia of dairy sciences. 2nd ed. Vol. 3. San Diego: Academic Press; 2011. p. 338–345.
- Kristensen C, Larsen LE, Trebbien R, Jensen HE. The avian influenza A virus receptor SA-α2,3-Gal is expressed in the porcine nasal mucosa sustaining the pig as a mixing vessel for new influenza viruses. Virus Res. 2024;340:
199304 . DOIPubMedGoogle Scholar - Bojar D, Meche L, Meng G, Eng W, Smith DF, Cummings RD, et al. A useful guide to lectin binding: machine-learning directed annotation of 57 unique lectin specificities. [PubMed]. ACS Chem Biol. 2022;17:2993–3012. DOIPubMedGoogle Scholar
- Spichtig V, Michaud J, Austin S. Determination of sialic acids in milks and milk-based products. Anal Biochem. 2010;405:28–40. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: August 09, 2024
Table of Contents – Volume 30, Number 9—September 2024
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Charlotte Kristensen, University of Copenhagen, Stigbøjlen 4, 1870 Frederiksberg C, Denmark
Top