Volume 31, Number 1—January 2025
Dispatch
Fatal Case of Crimean-Congo Hemorrhagic Fever, Portugal, 2024
Cite This Article
Citation for Media
Abstract
We report a fatal case of Crimean-Congo hemorrhagic fever in Portugal. An 83-year-old man, initially suspected of having Mediterranean spotted fever, was later confirmed to have Crimean-Congo hemorrhagic fever by the detection of viral genome in the patient's serum and the presence of specific IgM antibodies.
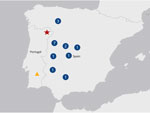
Crimean-Congo hemorrhagic fever (CCHF) is a potentially severe or fatal disease caused by CCHF virus (CCHFV; species Orthonairovirus haemorrhagiae), a tickborne virus of the family Nairoviridae, order Bunyavirales. CCHF has a broad geographic distribution that includes Africa, Asia, the Middle East, and some eastern and southern European countries (1). In Spain, the first cases were reported in 2016 in the province of Ávila, Castilla-León region (2). To date, a total of 17 confirmed cases have been documented in Spain, including 4 cases reported in 2024; the province of Salamanca reported 1 case in April, Toledo reported 1 case in July, Córdoba (unconfirmed but most likely) reported 1 case in July, and Cáceres reported 1 case in August (3). In Portugal, antibodies to CCHFV were first detected in 2 human serum samples in 1985. Of the 2 patients, 1 showed clinical signs and symptoms compatible with CCHF (4). Since 1985, CCHFV has not been detected in ticks or humans, although several studies have been conducted on ticks. Beginning in 2020, surveillance has been conducted within the national vector surveillance network, which systematically collects ticks throughout the country (5).
CCHFV is maintained by both vertical and horizontal transmission cycles involving ticks, mostly of the genus Hyalomma, and various species of wild and domestic mammals and birds that develop transient viremia without showing signs of disease (6). Human infection occurs through tick bites (or exposure to arthropod fluids) or direct contact with the secretions, fluids, or tissues of viremic animals and humans, especially in the absence of appropriate protective measures (7,8). Although nosocomial infections and outbreaks have been reported, the risk of acquiring CCHFV from exposure to infected biologic fluids appears to be lower than for other hemorrhagic viruses, such as Ebola (9). Asymptomatic CCHFV infections are thought to be common and may account for up to 90% of cases in hyperendemic areas (10). However, CCHFV can cause a severe or fatal clinical course; mortality rates have ranged from 3% to 40% (6,11). In this article, we report a clinically documented case of autochthonous CCHF in Portugal with a fatal outcome.
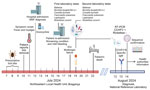
On July 12, 2024, an 83-year-old man was admitted to Bragança Public Hospital (Bragança, Portugal) with complaints of fever (39.5°C) and myalgia. The patient reported removing a tick on July 10 (Table 1) from the periumbilical region 1 day before the onset of symptoms on July 11. He lived in the district of Bragança, in a rural environment, and mentioned participation in outdoor social events and activities from June 29–July 7; he had no history of international travel (Figure 1). We made a presumptive diagnosis of Mediterranean spotted fever (MSF), and initiated treatment with doxycycline (200 mg/d) before discharge. However, on July 16, day 6 of symptoms, the patient was readmitted with gastrointestinal symptoms (nausea, vomiting, diarrhea) and persistent myalgia and fever. On physical examination, his temperature was 38.5°C, heart rate 125 beats/min, respiratory rate 25 breaths/min, and blood pressure 90/56 mm Hg; and he had a small periumbilical eschar and a petechial rash on his legs. Laboratory testing revealed thrombocytopenia, prolonged activated partial thromboplastin time, hypofibrinogenemia, elevated transaminases, lymphopenia, and hepatocellular cytolysis (Table 2). His condition gradually worsened, and gingival bleeding and epistaxis required transfusion. We treated the patient symptomatically: correction of hypovolemia and electrolyte imbalance caused by gastrointestinal disorders, correction of hemostasis (platelet transfusion and fibrinogen), and antimicrobial drug administration. On July 18, he was transferred to the intensive care unit because of multiple organ failure. We collected a second serum specimen on July 19 that tested negative for various tickborne pathogens. However, the specimen was positive for IgG against Rickettsia by using Rickettsia conorii IFA Slide (BIOCELL Diagnostics, https://biocelldx.com), which indicated past exposure to a spotted fever group Rickettsia. The patient died on July 22 (Figure 2).
The case was reviewed at a regular hospital medical meeting because of the unclear cause of death. Because of the recent CCHF cases in the neighboring regions in Spain, a day 9 serum sample from the patient was sent to the reference laboratory of the Portuguese National Institute of Health for CCHFV testing and PCR for Rickettsia spp. by using the Genesig PCR Kit (Primerdesign Ltd, https://www.genesig.com). The sample tested positive for CCHFV. We inactivated the sample in the Biosafety Level 3 facility by using the QIAamp Viral RNA Kit (QIAGEN, https://www.qiagen.com), followed by extraction under Biosafety Level 2 conditions. We conducted molecular testing by using the RealStar CCHFV RT-PCR Kit 1.0 (Altona Diagnostics, https://altona-diagnostics.com) and confirmed by using an in-house real-time reverse transcription PCR (RT-PCR) protocol (12). We purified a fragment (122 bp) from the small segment that was amplified by conventional RT-PCR by using the primers described previously (12) and conducted Sanger sequencing (GenBank accession no. PQ200212). Similarity searches within the GenBank dataset by using the BLAST algorithm (13) showed 100% similarity to CCHFV genotype III strains circulating in Spain (2014 and 2016) and Mauritania (1984). We tested for the presence of specific antibodies against CCHFV by using the Crimean-Congo fever virus Mosaic 2 immunofluorescence assay (EUROIMMUN, https://www.euroimmun.com) for IgM and IgG against the glycoprotein (GPC–glycoprotein precursor) and nucleoprotein antigens. The patient’s serum was positive for the IgM nucleoprotein-specific antigen at 128 titers (cutoff for positivity >16) (Figure 2).
We describe an autochthonous case of CCHF in Portugal in a deceased patient infected by a tick bite. The patient resided in Bragança, a district with a high incidence of MSF, and was initially misdiagnosed as having MSF because of overlapping epidemiologic, clinical, and laboratory features with CCHF (2,14). At admission on July 11, the patient showed no signs of severe illness. Of consequence, doxycycline, the standard antimicrobial for MSF, was administered, and the patient was then discharged. Of note, the patient had not traveled outside the district in the 2 weeks before symptom onset. Although CCHFV circulation has long been recognized in Portugal (4) and routine surveillance by RT-PCR is performed on Hyalomma spp. ticks collected throughout the country (5), the virus had not previously been detected by RT-PCR. All risk factors considered for CCHF infection in Portugal are similar to those known in Spain, including a favorable climate, the widespread presence of a tick vector, the country's geographic location along the migratory route of birds from CCHFV endemic areas, and proximity to Africa (15).
This case highlights the public health threat posed by CCHFV in Portugal, particularly because of the widespread distribution of Hyalomma ticks in the country. Increased vector surveillance and studies with more tick samples from ungulates, especially red deer, as well as serosurveys in wildlife and humans are urgently needed. Public awareness campaigns should focus on preventative behaviors for avoiding tick exposure, especially among outdoor enthusiasts and professionals in nature-related activities.
Although attention to CCHF is most often drawn by severe cases, the clinical manifestations might be mild and could go unnoticed by clinicians. This case emphasizes the importance of including CCHF in the differential diagnosis list of MSF. Because of the high biologic risk of CCHFV transmission and the biosafety conditions required for its handling, prompt risk assessment is critical for rapid diagnosis of suspected cases.
Dr. Zé-Zé, is a researcher at the INSA National Reference Laboratory for Vector-Borne Viruses, Águas de Moura, Portugal. Her interests include molecular diagnosis and research on arboviruses, hemorrhagic fever viruses and detection of emerging vector-borne viruses circulating in Portugal.
Acknowledgments
We thank Madalena Alves and the Clinical Pathology Department of the Northeast Local Health Unit for their invaluable collaboration in the clinical diagnosis, and the Emergency and Intensive Care Department teams for their exceptional patient care. We thank Maria Salomé Gomes for carrying out the serological testing, Patrícia Soares for generating the Iberia map in R, and the Innovation Unit, Human Genetics Department, National Institute of Health for technical support in Sanger sequencing.
This work was partially supported by the Portuguese Foundation for Science and Technology (grant nos. FCT/MCTES UIB/00211/2020, DOI 10.54499/UIDB/00211; FCT/MCTES UIP/00211/2020, DOI 10.54499/UIDP/00211; UIDB/04295/2020; and UIDP/04295/2020).
References
- Kuhn JH, Alkhovsky SV, Avšič-Županc T, Bergeron É, Burt F, Ergünay K, et al. ICTV virus taxonomy profile: Nairoviridae 2024. J Gen Virol. 2024;105:
001974 .PubMedGoogle Scholar - Negredo A, de la Calle-Prieto F, Palencia-Herrejón E, Mora-Rillo M, Astray-Mochales J, Sánchez-Seco MP, et al.; Crimean Congo Hemorrhagic Fever@Madrid Working Group. Autochthonous Crimean-Congo Hemorrhagic Fever in Spain. N Engl J Med. 2017;377:154–61. DOIPubMedGoogle Scholar
- ECDC – Surveillance and Disease data. Cases of Crimean–Congo haemorrhagic fever infected in the EU/EEA, 2013–present. [cited 2024 Oct 13]. https://www.ecdc.europa.eu/en/crimean-congo-haemorrhagic-fever/surveillance/cases-eu-since-2013
- Filipe AR, Calisher CH, Lazuick J. Antibodies to Congo-Crimean haemorrhagic fever, Dhori, Thogoto and Bhanja viruses in southern Portugal. Acta Virol. 1985;29:324–8.PubMedGoogle Scholar
- Centro de Estudos de Vetores e Doenças Infeciosas Doutor Francisco Cambournac. REVIVE 2023 report—Culicidae, Ixodidae and Phlebotomus: vector surveillance network [in Portuguese]. 2024. [cited 2024 Oct 13] http://hdl.handle.net/10400.18/9172
- Bente DA, Forrester NL, Watts DM, McAuley AJ, Whitehouse CA, Bray M. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Res. 2013;100:159–89. DOIPubMedGoogle Scholar
- Gunes T, Engin A, Poyraz O, Elaldi N, Kaya S, Dokmetas I, et al. Crimean-Congo hemorrhagic fever virus in high-risk population, Turkey. Emerg Infect Dis. 2009;15:461–4. DOIPubMedGoogle Scholar
- Whitehouse CA. Crimean-Congo hemorrhagic fever. Antiviral Res. 2004;64:145–60. DOIPubMedGoogle Scholar
- Leblebicioglu H, Sunbul M, Guner R, Bodur H, Bulut C, Duygu F, et al. Healthcare-associated Crimean-Congo haemorrhagic fever in Turkey, 2002-2014: a multicentre retrospective cross-sectional study. Clin Microbiol Infect. 2016;22:387.e1–4. DOIPubMedGoogle Scholar
- Bodur H, Akinci E, Ascioglu S, Öngürü P, Uyar Y. Subclinical infections with Crimean-Congo hemorrhagic fever virus, Turkey. Emerg Infect Dis. 2012;18:640–2. DOIPubMedGoogle Scholar
- Sidira P, Maltezou HC, Haidich AB, Papa A. Seroepidemiological study of Crimean-Congo haemorrhagic fever in Greece, 2009-2010. Clin Microbiol Infect. 2012;18:E16–9. DOIPubMedGoogle Scholar
- Atkinson B, Chamberlain J, Logue CH, Cook N, Bruce C, Dowall SD, et al. Development of a real-time RT-PCR assay for the detection of Crimean-Congo hemorrhagic fever virus. Vector Borne Zoonotic Dis. 2012;12:786–93. DOIPubMedGoogle Scholar
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10. DOIPubMedGoogle Scholar
- de Sousa R, Nóbrega SD, Bacellar F, Torgal J. Mediterranean spotted fever in Portugal: risk factors for fatal outcome in 105 hospitalized patients. Ann N Y Acad Sci. 2003;990:285–94. DOIPubMedGoogle Scholar
- Lorenzo Juanes HM, Carbonell C, Sendra BF, López-Bernus A, Bahamonde A, Orfao A, et al. Crimean-Congo hemorrhagic fever, Spain, 2013–2021. Emerg Infect Dis. 2023;29:252–9. DOIPubMedGoogle Scholar
Figures
Tables
Cite This ArticleOriginal Publication Date: December 05, 2024
1These first authors contributed equally to this article.
Table of Contents – Volume 31, Number 1—January 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Líbia Zé-Zé, Instituto Nacional de Saúde Doutor Ricardo Jorge, Avenida da Liberdade 5, 2965-575 Águas de Moura, Portugal
Top