Volume 31, Number 10—October 2025
Research
Effect of Seasonal Influenza Vaccines on Avian Influenza A(H5N1) Clade 2.3.4.4b Virus Infection in Ferrets
Figure 4
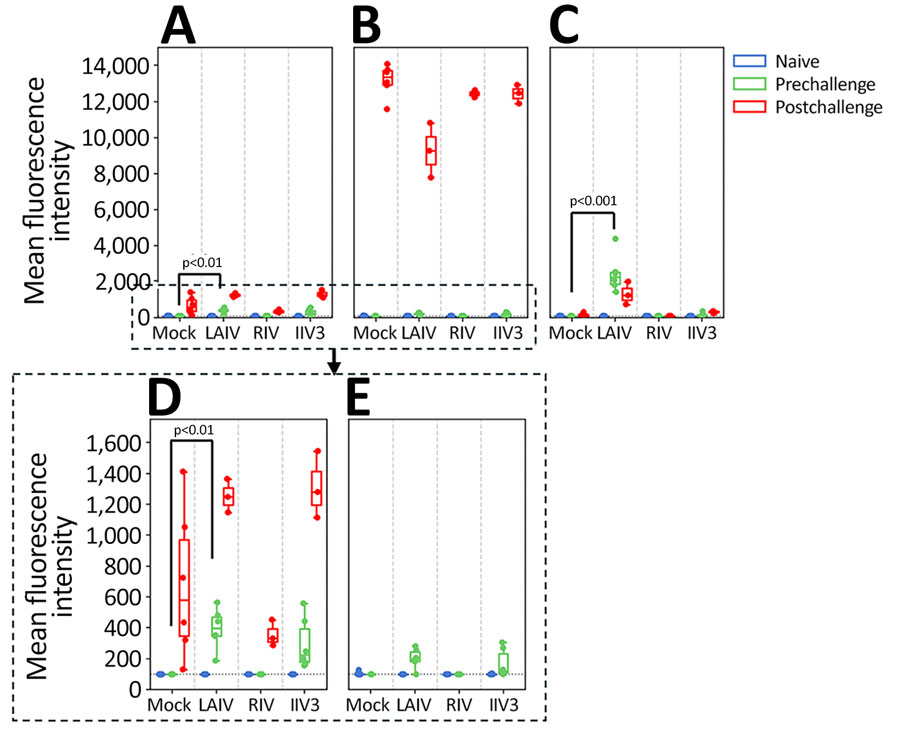
Figure 4. Neuraminidase antibody titers measured by using multiplex influenza antibody detection assay in study of the effect of seasonal influenza vaccines on influenza A(H5N1) clade 2.3.4.4b virus infection in ferrets. n = 6 ferrets per vaccinated group, n = 12 ferrets in the mock vaccinated group. A) Neuraminidase N1 from A/Wisconsin/67/2022(H1N1pdm09). B) Neuraminidase N1 from A/Texas/37/2024(H5N1). C) Neuraminidase N2 from A/Massachusetts/18/2022(H3N2). D) Expanded view of neuraminidase N1 from A/Wisconsin/67/2022(H1N1pdm09) to more clearly show the cross-reactive responses postvaccination. E) Expanded view of neuraminidase N1 from A/Texas/37/2024(H5N1) to more clearly show the cross-reactive responses postvaccination. Colored dots indicate individual values, horizontal lines within boxes represent geometric mean titers, box tops and bottoms indicate interquartile ranges, and error bars indicate limits of the distribution. Dotted gray horizontal line indicates limit of detection. IIV3, Fluarix trivalent inactivated influenza vaccine (GlaxoSmithKline Biologicals, https://www.gsk.com); LAIV, FluMist live attenuated influenza vaccine (AstraZeneca, https://www.astrazeneca.com); RIV, Flublok recombinant influenza vaccine (Sanofi, https://www.sanofi.com).
1These first authors contributed equally to this article.