Volume 31, Number 12—December 2025
Dispatch
Human Infection by Zoonotic Eye Fluke Philophthalmus lacrymosus, South America
Abstract
We report a case of severe conjunctivitis in a traveler infected with a Philophthalmus lacrymosus eye fluke, probably acquired on the Galápagos Islands in Ecuador. This zoonotic parasite is endemic in Brazil and Venezuela, where it has been reported in birds and capybaras.
Philophthalmus spp. parasites are cosmopolitan digeneans, typically inhabiting the conjunctival sac of waterbirds. The trematodes, known as avian eye flukes, have a complex lifecycle, which includes freshwater and marine snails as intermediate hosts and waterbirds as final hosts. Birds are infected by ingestion of infective metacercariae that are encysted on aquatic plants (1). After thermally triggered excystation in the pharynx, the parasite migrates through the lacrimal ducts to the orbital cavity. Ocular infections of mammals have been reported in capybaras (Hydrochoerus hydrochaeris) from Brazil, Galapagos sea lions (Zalophus wollebaeki) in Ecuador, and rarely other mammals, including humans (2–6). The mode of infection in nonavian hosts is uncertain; both ingestion and direct contact with cercariae or metacercariae have been proposed (2,7).
Globally, >50 nominal Philophthalmus spp. trematodes have been described; however, recent evidence suggests that only ≈10 species are valid (1,5). In South America, Philophthalmus spp. flukes have been reported from Brazil, Peru, Venezuela, and Ecuador’s Galápagos Islands (1,3,8,9). Further geographic spread of Philophthalmus spp. trematodes by invasive snail species is probable (9,10).
Since 1939, a total of 12 human philophthalmiasis cases have been published. Infections were acquired in Asia, Europe, and North America and mostly identified to the genus level, but cases compatible with philophthalmiasis already were known in the 19th Century (Appendix 1). We describe a case of philophthalmiasis caused by a Philophthalmus lacrymosus eye fluke in a traveler from Europe, probably acquired on the Galápagos Islands.
A 26-year-old woman from England sought care in Santiago, Chile, for a 9-day history of intense pain, swelling, and a moving foreign body sensation in her right eye. Before symptom onset, she had visited Colombia (4-week stay); Ecuador, including Galápagos Islands (2.5-week stay); and Peru (1-week stay). Ocular examination showed eyelid edema, intense chemosis and follicular reaction of the inferior fornix, and superior tarsal conjunctiva. Results of cornea examination, anterior segment findings, and fundus examination were unremarkable. After a thorough examination, we removed an elongated mobile structure located on the upper tarsal conjunctiva by using a moist cotton swab. After removal, the foreign body sensation disappeared. Follow-up over the following weeks showed a complete recovery without complications.
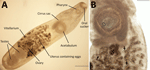
We performed detailed morphologic studies of the extracted structure on a temporary wet mount by using an Olympus SZ61 stereo microscope (https://www.olympus-global.com), an Olympus DP22 digital camera, and Olympus cellSens software version 2.3 (https://evidentscientific.com). Our analyses confirmed the specimen as a P. lacrymosus fluke (Figure 1). The single mature ovigerous specimen had an elongated oval shape, smooth surface, no spines, and constriction at the level of the ventral sucker. Maximum width was always posterior to ventral sucker, oral sucker subterminal, pharynx muscular, esophagus bifurcating posteriorly to pharynx, and ceca extending to posterior margin of posterior testis. The acetabulum was larger than the oral sucker and preequatorial; testes were in tandem, smooth and spherical, located in the posterior portion of the body, in an intercecal position; cirrus sac was elongated, slightly surpassing posterior margin of acetabulum (cirrus not visible), and genital pore median, at level of cecal bifurcation. Ovary was spherical, located pretesticular in the intercecal area; the uterus was long and coiled, occupying the space between the ventral sucker and the level of anterior margin of the anterior testis; the vitellarium was follicular and eggs nonoperculated, containing a fully formed miracidium with a dark eyespot in most eggs. We morphometrically compared the sample with previously reported P. lacrymosus specimens (Appendix 1).
We confirmed species diagnosis by molecular analyses using PCR and bidirectional Sanger sequencing of nuclear internal transcribed spacer (ITS) 2 and mitochondrial cytochrome c oxidase I (Cox1) (Appendix 2). We compared amplicons with sequences from GenBank and Philophthalmus zalophi ocular trematodes from Galápagos sea lions.
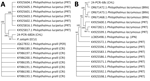
An 861-bp ITS-2 consensus sequence from the human sample was identical to the consensus P. zalophi sequence (M.J. Yabsley, unpub. data) and showed 98.6% identity with P. lacrymosus and 95.9% identity with P. lucipetus, both fluke species found in gulls (Larus spp.) from Portugal, and 95.6% identity with P. gralli sequences from invasive red-rimmed melania snails (Melanoides tuberculata) from Costa Rica and small passerines from Peru (Figure 2). The 396-bp Cox1 consensus sequence had 99.73% (single transition) and 99.45% identity with P. lacrymosus sequences from kelp gulls (Larus dominicanus) in Brazil, whereas similarities with P. lacrymosus sequences from Portugal were 91.90%–92.15%. We observed identity values ranging from 87.34% to 87.09% with P. lucipetus sequences from Portugal. An 87.12% identity was shared with a specimen annotated as Philopththalmus sp., which was isolated from a Japanese snail (Semisulcospira libertina) (Figure 2). We constructed additional Bayesian-inferred phylogenetic trees from 17 ITS-2 sequences and 16 Cox1 sequences (Appendix 1 Figure).
The epidemiology of human philophthalmiasis is poorly understood. Cases have been reported from Asia, Europe, and North America (Appendix 1 Table 1). Some reports suggest infection by direct inoculation of metacercariae during swimming (Appendix 1), whereas others suggest oral ingestion of metacercaria with food or direct inoculation during food preparation (Appendix 1). Of note, the parasite can survive for several months in the human host (Appendix 1). Nearly all cases were caused by single worms and involved unilateral irritation, sensation of a foreign body, and conjunctivitis. Vision impairment has not been reported, except for historical cases in the 18th Century with high worm loads (Appendix 1).
The number of species causing human philophthalmiasis is uncertain, given that most extracted worms were identified only to genus. In North America, the P. lacrymosus fluke (recorded as P. lacrimosus) was diagnosed in a human case from Mexico (11). In South America, P. lacrymosus flukes have exclusively been diagnosed in waterbirds in Brazil and Venezuela and capybaras in Brazil (5). The patient reported here had only direct contact with natural water environments on the Galápagos Islands, where P. zalophi, a new fluke species defined by morphologic criteria, has been reported in Galapagos sea lions (3,4). Considering that Philophthalmus parasites can inhabit the human eye for several months, a previous case from Ohio, USA, might plausibly have also been acquired on the Galápagos Islands, which the patient had visited 5 months before symptom onset (12). Marine snails of the Batillariidae family, present on the Galápagos Islands, could serve as potential intermediate hosts; that family includes the West Indian false cerith (Lampanella minima), a known intermediate host of P. lacrymosus flukes (13,14).
The patient’s exposure on Galápagos Islands remains inferential, requiring studies of infected intermediate hosts or environmental larval stages. However, our molecular data indicate that P. zalophi flukes from Galápagos might be conspecific with P. lacrymosus flukes. Probable spillover of P. lacrymosus flukes from a bird host to sea lions could explain morphologic differences, which can occur during adaptation to the mammalian host as reported in capybaras (2). Similarly, host-related plasticity or different development stages could explain certain morphologic deviations of our sample from previous P. lacrymosus specimens (Appendix 1). The specimen we report shared several traits with P. zalophi flukes, including a similar oral sucker to pharynx ratio and a body length <6 mm.
Further comparative genomic analyses are required to clarify taxonomic uncertainty of Philophthalmus spp. flukes infecting humans, as recently shown in Japan (15). The P. lacrymosus species might be paraphylectic or represent a complex of geographically and host-related lineages with South American isolates forming a genetically cohesive clade that is taxonomically distinct from forms of P. lacrymosus flukes in Europe and Asia.
In conclusion, our clinical and epidemiologic findings show that the zoonotic eye fluke P. lacrymosus can infect humans in South America. The findings also suggest that the parasite might be endemic on the Galápagos Islands in Ecuador.
Dr. Weitzel is a tropical medicine and parasitology expert at Clínica Alemana, Universidad del Desarrollo, Santiago, Chile. His research interests include vectorborne, parasitic, and travel-associated infections.
Acknowledgment
We thank James Flowers for the field work on the Galápagos Islands and Armin Araya and Kayla Garrett for laboratory assistance.
References
- Nollen PM, Kanev I. The taxonomy and biology of philophthalmid eyeflukes. Adv Parasitol. 1995;36:205–69. DOIGoogle Scholar
- Pinto RM, dos Santos LC, Tortelly R, Menezes RC, de Moraes W, Juvenal JC, et al. Pathology and first report of natural infections of the eye trematode Philophthalmus lachrymosus Braun, 1902 (Digenea, Philophthalmidae) in a non-human mammalian host. Mem Inst Oswaldo Cruz. 2005;100:579–83.PubMedGoogle Scholar
- Dailey M, Ellin R, Parás A. First report of parasites from pinnipeds in the Galapagos Islands, Ecuador, with a description of a new species of Philophthalmus (Digenea: Philophthalmidae). J Parasitol. 2005;91:614–7.PubMedGoogle Scholar
- Phillips BE, Páez-Rosas D, Flowers JR, Cullen JM, Law JM, Colitz C, et al. Evaluation of the ophthalmologic disease and histopthologic effects due to the ocular trematode Philophthalmus zalophi on juvenile Galápagos sea lions (Zalophus wollebaeki). J Zoo Wildl Med. 2018;49:581–90.PubMedGoogle Scholar
- Hernández DL, Somma AT, Steuernagel A, Vieira TSWJ, Moore B, Reifur L, et al. A molecular phylogenetic study of the eye fluke Philophthalmus lacrymosus (Trematoda: Philophthalmidae) found in Larus dominicanus (Aves: Laridae) from Brazil. Acta Parasitol. 2024;69:1027–34.PubMedGoogle Scholar
- Meise K, Garcia-Parra C. Behavioral and environmental correlates of Philophthalmus zalophi infections and their impact on survival in juvenile Galapagos sea lions. Mar Biol. 2015;162:2107–17. DOIGoogle Scholar
- Rajapakse RD, Wijerathne KMS, S de Wijesundera M. Ocular infection with an avian trematode (Philophthalmus sp). Ceylon Med J. 2009;54:128–9.PubMedGoogle Scholar
- Pulido-Murillo EA, Tkach VV, Pinto HA. The life cycle of Philophthalmus aylacostoma n. sp. (Trematoda: Philophthalmidae), a new eye fluke species transmitted by Aylacostoma spp. (Gastropoda: Thiaridae) in Brazil. Parasitol Res. 2022;121:933–44.PubMedGoogle Scholar
- Literák I, Heneberg P, Sitko J, Wetzel EJ, Cardenas Callirgos JM, Čapek M, et al. Eye trematode infection in small passerines in Peru caused by Philophthalmus lucipetus, an agent with a zoonotic potential spread by an invasive freshwater snail. Parasitol Int. 2013;62:390–6.PubMedGoogle Scholar
- Metz DCG, Turner AV, Nelson AP, Hechinger RF. Potential for emergence of foodborne trematodiases transmitted by an introduced snail (Melanoides tuberculata) in California and elsewhere in the United States. J Infect Dis. 2023;227:183–92.PubMedGoogle Scholar
- Lamothe-Argumedo R, Diaz-Camacho SP, Nawa Y. The first human case in Mexico of conjunctivitis caused by the avian parasite, Philophthalmus lacrimosus. J Parasitol. 2003;89:183–5.PubMedGoogle Scholar
- Gutierrez Y, Grossniklaus HE, Annable WL. Human conjunctivitis caused by the bird parasite Philophthalmus. Am J Ophthalmol. 1987;104:417–9.PubMedGoogle Scholar
- Heneberg P, Casero M, Waap H, Sitko J, Azevedo F, Těšínský M, et al. An outbreak of philophthalmosis in Larus michahellis and Larus fuscus gulls in Iberian Peninsula. Parasitol Int. 2018;67:253–61.PubMedGoogle Scholar
- Charles Darwin Foundation. Galapagos species database [cited 2025 Jul 12]. https://datazone.darwinfoundation.org/en/checklist/?species=9355
- Sasaki M, Miura O, Nakao M. Philophthalmus hechingeri n. sp. (Digena: Philophthamidae), a human-infecting eye fluke from the Asian mud snail, Batillaria attramentaria. J Parasitol. 2022;108:44–52.PubMedGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: December 12, 2025
1These authors contributed equally to this article.
Table of Contents – Volume 31, Number 12—December 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Thomas Weitzel, Laboratorio Clínico, Clínica Alemana, Av. Vitacura 5951, Santiago, Chile
Top