Volume 31, Number 7—July 2025
Dispatch
Multisystemic Disease and Septicemia Caused by Presumptive Burkholderia pseudomallei in American Quarter Horse, Florida, USA
Abstract
We report a presumptive case of melioidosis caused by an atypical Burkholderia pseudomallei serotype in an American quarter horse in north-central Florida, USA, through archived formalin-fixed paraffin-embedded specimens dating back to 2006. This case underscores the potential pathologic impact of emergent B. pseudomallei in the Gulf region of the United States.
Burkholderia pseudomallei is a gram-negative bacterium and the causative agent of the deadly disease melioidosis (1). This pathogen is a saprophytic bacillus distributed in the soil and water of tropical and subtropical environments. Regions where melioidosis is endemic include most of Southeast Asia, South America, the Caribbean, and northern Australia (2). Recently, B. pseudomallei was isolated from 3 unrelated patients from Mississippi, USA, who had no travel history to a melioidosis-endemic country (3–5). Those patients demonstrated symptoms consistent with melioidosis. Genetically similar organisms were isolated from the local soil and water, suggesting environmental transmission (3).
In addition to humans, many animal species, including horses, have been identified as susceptible to melioidosis (6,7). Clinical signs associated with melioidosis in animals mimic those of other virulent bacterial diseases and include lethargy, purulent nasal discharge, multiorgan abscesses, septicemia, and death by acute or chronic disease (8). Glanders, caused by the closely related B. mallei, can also cause similar clinical signs. B. mallei does not survive in the soil but can infect many species through animal–animal or zoonotic infection (8). Glanders has long been eradicated in the United States (8); however, this pathogen remains endemic in some regions of the Middle East, Asia, Africa, and Central and South America (8). Diagnosing either entity within the United States is critical because of the zoonotic potential of both organisms and the possible implications for public health. In addition, horses and livestock can be sentinel species for the environmental presence of B. pseudomallei, suggesting environmental contamination and posing risks to animals and humans. Predictive modeling studies indicate that B. pseudomallei might be ubiquitous throughout tropical and subtropical areas worldwide, including the southern United States (4,5).
The primary routes of B. pseudomallei infection are ingestion, inhalation, and percutaneous inoculation (1,9). The incidence of melioidosis increases dramatically after heavy rainfall (9). In addition, B. pseudomallei is classified as a category B bioterrorism bacterium and a Tier 1 (top tier) agent by the Centers for Disease Control and Prevention and Tier 1 by the US Department of Agriculture (1). Moreover, B. pseudomallei is highly resistant to antimicrobial drugs commonly used to treat sepsis in humans and animals, and an effective vaccine has not been approved (1). Furthermore, in apparently successfully treated humans and animals, relapses are common and precede development of chronic melioidosis (9). This article discusses a presumptive case of B. pseudomallei causing melioidosis-like diseases in an American quarter horse (Equus caballus) in Florida, USA.
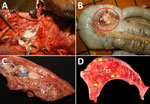
In 2006, an 8-year-old quarter horse gelding with a left retropharyngeal abscess was seen at the University of Florida Veterinary Medical Center (Gainesville, Florida, USA). Clinical examination and radiographs revealed a well-circumscribed 20 × 10 × 18-cm round soft tissue mass caudal to the ramus of the mandible with displacement of the left guttural pouch. Other examination findings included diffuse interstitial pneumonia, multiple cutaneous ulcers on the dorsal midline, anterior uveitis in the right eye, enlarged mesenteric lymph nodes, and an aneurysm of the right renal artery. Because of the animal’s declining clinical condition, it was humanely euthanized. At necropsy examination, gross findings included a large, soft, round abscess in the retropharyngeal space, compressing the guttural pouch with a draining tract into the epidermis (Figure 1, panel A). The mesenteric lymph nodes were diffusely enlarged, ranging from 2.0 to 6.0 cm in diameter, with purulent material and hemorrhage (Figure 1, panel B). Other gross findings included diffuse interstitial pneumonia with multifocal 2.0 × 2.0 × 2.0-cm peribronchiolar abscesses (Figure 1 panel C). Small 1.0 × 1.0 × 1.0-cm randomly scattered areas of necrosis were multifocally scattered in the liver (Figure 1, panel D); anterior uveitis was present in the right eye.
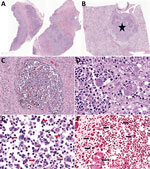
Tissue specimens were fixed in 10% neutral buffered formalin, processed routinely, and embedded in paraffin. Paraffin-embedded sections were cut 4 μm thick and examined after staining with hematoxylin and eosin. We performed histochemical Gram stain to screen for bacteria. Histologic sections from the affected submandibular lymph node (Figure 2, panel A), lung (Figure 2, panel B), and mesentery revealed multifocal to coalescing abscesses and pyogranulomas containing necrotic cellular debris and degenerative neutrophils mixed with hemosiderophages (Figure 2, panels C–E). Numerous macrophages and neutrophils contained intracytoplasmic 1–2 µm gram-negative bacilli (Figure 2, panel F).
Postmortem aerobic culture of the retropharyngeal and mesenteric lymph nodes revealed pure B. cepacia complex growth. A fatty acid analysis using gas chromatography of the bacterial isolate supported this finding. B. cepacia complex is a heterogeneous group of bacteria from the Burkholderia genus that typically causes opportunistic infections in immunocompromised hosts. Disease outbreaks often occur in the hospital setting through contaminated medical devices or in patients with chronic respiratory diseases, such as cystic fibrosis (10).
The clinical manifestations of this case were unusual for B. cepacia complex and more consistent with the clinical course of acute, highly pathogenic Burkholderia species. We suspect that the organism was misidentified. The precise methodology of the original diagnosis was not reported in the case documents and is unknown to the authors of this report. Current biochemical systems in historically nonendemic areas often mistakenly identify B. pseudomallei strains as members of B. cepacia complex (11). Our team concluded that further investigation was necessary. Unfortunately, because of DNA fragmentation during routine tissue processing for histopathology, attempts at isolating bacterial DNA from the formalin-fixed paraffin-embedded tissue samples were unsuccessful. A limitation of this study is the inability to extract bacterial DNA from the tissue block, preventing definitive identification of B. pseudomallei.
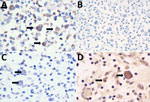
B. pseudomallei employs a network of polysaccharides, including capsular polysaccharides (CPS) and lipopolysaccharides (LPS), to enhance virulence and immune evasion (12,13). A panel of monoclonal antibodies targeting B. pseudomallei and B. mallei CPS (4C4), typical LPS O-Ag serotype A (4C7), atypical LPS O-Ag serotype B and its variant B2 (3A2, 5B4), and B. mallei LPS O-Ag (3D11) were used (13). The secondary antibody was biotinylated goat anti-mouse IgG (1:200 dilution). Intrahistiocytic bacilli were immunopositive for 4C4, 3A2, and 5B4 and immunonegative for 4C7 and 3D11 (Figure 3). We tested 19 different strains of B. cepacia complex and demonstrated no cross-reactivity to 4C4, 4C7, 3A2, and 5B4 on Western blot analysis (Table). Previous studies have shown B. pseudomallei cross-reactivity by some B. cepacia complex strains to B. pseudomallei–like CPS-specific antibodies (4C4) (14) and B. mallei to monoclonal antibodies to typical type A LPS (4C7); however, cross-reactivity of either species to B. pseudomallei atypical type B LPS (3A2 and 5B4) has not been reported, and cross-reactivity was not noted in our experiments (15). The immunohistochemistry and Western blot results suggest infection with B. pseudomallei with atypical O-Ag type B or B2.
This case report describes the histomorphology and immunohistochemical identification of an atypical serotype of B. pseudomallei in a horse with clinical signs consistent with melioidosis. Although type A is the most common LPS O-Ag type of B. pseudomallei, accounting for most infections, types B and B2 are found more frequently in Australia (13). The route of exposure and travel history of this horse is unknown. On the basis of the immunohistochemistry results in this case, we conclude that the initial culture and biochemical analysis misidentified B. cepacia complex. Western blot assay analysis of purified LPS from numerous bacteria in the B. cepacia complex failed to highlight any of the mentioned monoclonal antibodies. This case underscores the potential pathological effects of B. pseudomallei in horses and other animals in the United States, emphasizing the need for increased awareness and understanding of its emergence as a potential pathogen in diverse species.
Dr. Thornton is a US Army Veterinary Corps Officer and a diplomate of the American College of Veterinary Pathologists. He is currently a PhD student at the University of Florida College of Veterinary Medicine in the Department of Infectious Diseases and Immunology, studying Burkholderia pseudomallei and B. mallei and their potential impact on the biosecurity in the United States.
Acknowledgments
We sincerely appreciate Puttawat Suphaprueksapong, Sintara Pumin, and Pacharapong Khrongsee for their invaluable assistance and contributions to this case study. We would also like to acknowledge and thank the technical and support staff at the University of Florida College of Veterinary Medicine Histopathology Laboratory, particularly Ana Herrera and Lindsay Vail, for their assistance in providing outstanding histology, histochemistry, and immunohistochemistry staining services.
This study was conducted in strict accordance with ethical principles and guidelines to ensure the highest standards of integrity, accountability, and respect for the animal subject. Because this study was a retrospective analysis of formalin-fixed paraffin-embedded tissue blocks and no live animals were used, this work is exempt from review by the University of Florida Institutional Animal Care and Use Committee.
J.J.T. is funded by a Department of Defense Long-Term Health and Education Training Program scholarship program. The views expressed in this article are those of the author and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or US Government.
References
- Wiersinga W, Virk H, Torres AG, Currie BJ, Peacock SJ, Dance DAB, et al. Melioidosis. Nat Rev Dis Primers. 2018;4:17108. DOIPubMedGoogle Scholar
- Limmathurotsakul D, Golding N, Dance DA, Messina JP, Pigott DM, Moyes CL, et al. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol. 2016;1:15008. DOIGoogle Scholar
- Centers for Disease Control and Prevention. Melioidosis locally endemic in areas of the Mississippi Gulf Coast after Burkholderia pseudomallei isolated in soil and water and linked to two cases—Mississippi, 2020 and 2022 [cited 2024 Jun 15]. https://emergency.cdc.gov/han/2022/han00470.asp
- Petras JK, Elrod MG, Ty MC, Dawson P, O’Laughlin K, Gee JE, et al. Locally acquired melioidosis linked to environment—Mississippi, 2020–2023. N Engl J Med. 2023;389:2355–62. DOIPubMedGoogle Scholar
- Torres AG. The public health significance of finding autochthonous melioidosis cases in the continental United States. PLoS Negl Trop Dis. 2023;17:
e0011550 . DOIPubMedGoogle Scholar - Ladds PW, Thomas AD, Pott B. Melioidosis with acute meningoencephalomyelitis in a horse. Aust Vet J. 1981;57:36–8. DOIPubMedGoogle Scholar
- Choy JL, Mayo M, Janmaat A, Currie BJ. Animal melioidosis in Australia. Acta Trop. 2000;74:153–8. DOIPubMedGoogle Scholar
- Khan I, Wieler LH, Melzer F, Elschner MC, Muhammad G, Ali S, et al. Glanders in animals: a review on epidemiology, clinical presentation, diagnosis and countermeasures. Transbound Emerg Dis. 2013;60:204–21. DOIPubMedGoogle Scholar
- Chomkatekaew C, Boonklang P, Sangphukieo A, Chewapreecha C. An evolutionary arms race between Burkholderia pseudomallei and host immune system: what do we know? Front Microbiol. 2021;11:
612568 . DOIPubMedGoogle Scholar - Łagowski D, Nowicka B, Nowakiewicz A, Polkowska I, Gnat S. Unusual penile prolapse with an infectious background caused by the Burkholderia cepacia complex in a stallion. J Equine Vet Sci. 2021;97:
103353 . DOIPubMedGoogle Scholar - Zakharova IB, Lopasteyskaya YA, Toporkov AV, Viktorov DV. Influence of biochemical features of Burkholderia pseudomallei strains on identification reliability by Vitek 2 system. J Glob Infect Dis. 2018;10:7–10. DOIPubMedGoogle Scholar
- Nualnoi T, Norris MH, Tuanyok A, Brett PJ, Burtnick MN, Keim PS, et al. Development of immunoassays for Burkholderia pseudomallei typical and atypical lipopolysaccharide strain typing. Am J Trop Med Hyg. 2017;96:358–67. DOIPubMedGoogle Scholar
- Tuanyok A, Stone JK, Mayo M, Kaestli M, Gruendike J, Georgia S, et al. The genetic and molecular basis of O-antigenic diversity in Burkholderia pseudomallei lipopolysaccharide. PLoS Negl Trop Dis. 2012;6:
e1453 . DOIPubMedGoogle Scholar - Burtnick MN, Dance DAB, Vongsouvath M, Newton PN, Dittrich S, Sendouangphachanh A, et al. Identification of Burkholderia cepacia strains that express a Burkholderia pseudomallei-like capsular polysaccharide. Microbiol Spectr. 2024;12:
e0332123 . DOIPubMedGoogle Scholar - Songsri J, Kinoshita Y, Kwanhian W, Wisessombat S, Tangpong J, Rahman-Khan MS, et al. Cross-reactivity of latex agglutination assay complicates the identification of Burkholderia pseudomallei from soil. FEMS Microbiol Lett. 2018;365:365. DOIPubMedGoogle Scholar
Figures
Table
Cite This ArticleOriginal Publication Date: June 17, 2025
Table of Contents – Volume 31, Number 7—July 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Jason Thornton, University of Florida College of Veterinary Medicine, 1945 SW 16th Ave, PO Box 11880, Gainesville, FL 32608, USA
Top