Volume 31, Number 8—August 2025
Online Report
Optimal Timing for Expanding Diagnostic Laboratories, South Korea
Figure 2
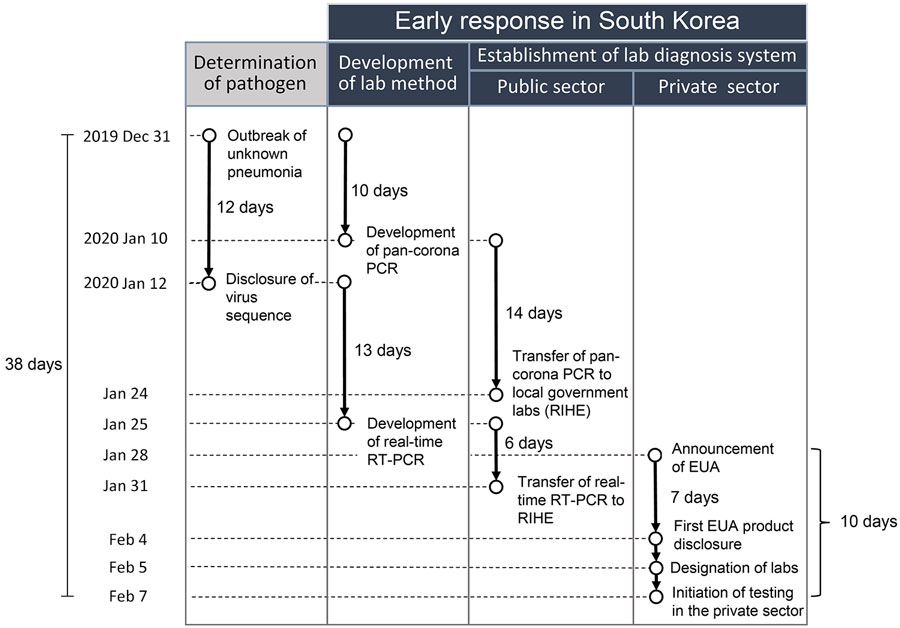
Figure 2. Flowchart of early diagnostic laboratory response to COVID-19 in South Korea in 2020. EUA, emergency use authorization; lab, laboratory; RIHE, Research Institute of Health and Environments; RT-PCR, reverse transcription PCR.
Page created: June 23, 2025
Page updated: July 22, 2025
Page reviewed: July 22, 2025
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.