Volume 31, Number 8—August 2025
Online Report
Optimal Timing for Expanding Diagnostic Laboratories, South Korea
Figure 4
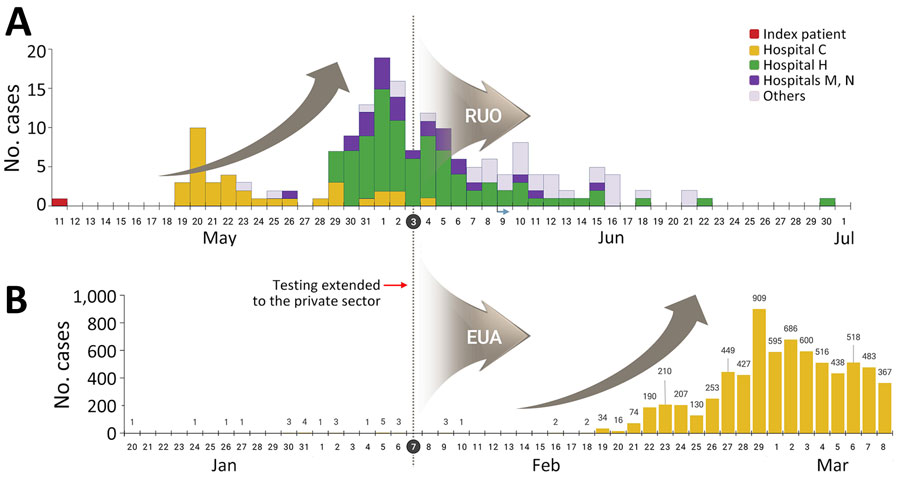
Figure 4. Diagnostic laboratory expansion for Middle East respiratory syndrome (MERS) and COVID-19, South Korea. Number of MERS cases during May–July 2015 (A) and number of COVID-19 cases during January–March 2020 (B) were compared. Red arrow and dotted vertical lines indicate when testing was expanded to include private medical testing laboratories. Gray curved arrows indicate when testing was conducted using RUO-approved or EUA-approved reagents. RUOs were used to implement new MERS diagnostic tests, and EUAs were used for novel COVID-19 diagnostic tests. EUA, emergency use authorization; RUO, research-use only.
Page created: June 23, 2025
Page updated: July 22, 2025
Page reviewed: July 22, 2025
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the U.S. Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the authors' affiliated institutions. Use of trade names is for identification only and does not imply endorsement by any of the groups named above.