Volume 31, Number 8—August 2025
Research Letter
Molecular Characterization of Echinococcus vogeli from Human Case, Colombia, 2024
Abstract
In Colombia, 35 confirmed cases of neotropical polycystic echinococcosis were reported during 1978–2018. In most cases, Echinococcus vogeli was identified by means of morphologic identification. We describe a case of E. vogeli echinococcosis in a woman, diagnosed through PCR, mitochondrial DNA sequencing, and molecular characterization.
Human echinococcosis (also known as hydatidosis) is a zoonotic neglected disease caused by infection of cestode larval form of Echinococcus spp. tapeworms (1,2). At least 4 Echinococcus species are recognized as causes of human disease and have relevance in public health: E. granulosus causes cystic echinococcosis and is a cosmopolitan species; E. multilocularis produces alveolar echinococcosis and predominates in the northern hemisphere; and E. vogeli causes neotropical polycystic and E. oligarthus unicystic echinococcosis, both confined to tropical zones in Central and South America (1,2). Neotropical polycystic echinococcosis (NPE) affects mostly persons living in rural and sylvatic regions where the cycle of the E. vogeli tapeworm involves the paca (Cuniculus paca) as the intermediate host and the bush dog (Speothus venaticus) as the natural final host (2,3). Nevertheless, a proposed domiciliary transmission cycle posits that human infection occurs incidentally through fecal contamination by domestic hunting dogs (alternative final host) after they fed on paca viscera (2,4).
In Colombia, 35 confirmed cases of NPE were reported during 1978–2018 (2,5–7). In most of them, Echinococcus spp. infection was identified by means of morphologic identification (2,5,6); in 1 case, the 2018 report, molecular detection identified the E. vogeli cytochrome c oxidase subunit 1 (cox1) mitochondrial gene without molecular characterization (7). Here, we present a case of E. vogeli echinococcosis in a woman in Colombia diagnosed through PCR, sequencing mitochondrial DNA, and molecular characterization. We obtained written consent from the patient to report on her case.
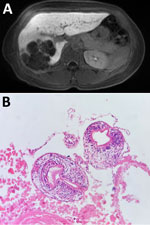
An otherwise healthy 50-year-old woman sought care at the emergency department of the Hospital Militar Central (Bogotá, Colombia) on September 23, 2024, after 8 days of epigastric and right hypochondrium pain; she did not have jaundice or other symptoms. As a child, she had lived in a rural region of Cesar Department (Colombian Caribbean region); she saw pacas often and always had kept domestic hunting dogs. Physical examination revealed a mild tenderness in right hypochondrium without peritoneal irritation signs. Serum liver function tests were without abnormality. Abdominal magnetic resonance imaging showed hypodense, round polycystic vesicles, replacing right liver parenchyma with predominant peripheral calcifications and fat content (Figure 1, panel A). Differential diagnoses were mucinous cystic neoplasm, hepatic liposarcoma, and hydatid cysts. We performed a total right segmental liver resection and cholecystectomy. The histopathological results of surgical liver specimen showed multiple cysts with Echinococcus protoscoleces (Figure 1, panel B). The patient received albendazole (200 mg 2×/d) for 1 month and was discharged.
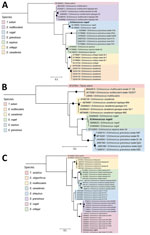
We performed PCR on the liver histopathological sample, targeting the cytochrome b (Cob), 12S rRNA, and cox1 genes, confirming the presence of Echinococcus sp. We sequenced the amplicons using Oxford Nanopore MinION (Oxford Nanopore Technologies, https://nanoporetech.com) and used those sequences for phylogenetic reconstruction. Phylogenetic analysis showed that the sequences we obtained of the 3 genes clustered with E. vogeli sequences downloaded from GenBank (Figure 2). Sequences from this study were deposited in GenBank (accession nos. PV243336, PV243987, and SUB15312175).
The clinical characteristics of NPE in patients depend on the location of the metacestode as well as the extent of invasion of tissues (2). The liver is the most frequently affected organ (2). Metacestodes could be found in the liver alone or with vesicles situated in the abdomen, in the liver and the lungs or pleural cavities, or only as calcified vesicles in the liver (2). Other organs involved included the diaphragm, spleen, pancreas, omentum, mesenteries, rectovesical pouch, ovaries, uterus, abdominal wall, psoas muscle, and vertebra (2). Before surgery, patients often receive misdiagnosis with a variety of disorders, including hepatic tumor, abscess, cirrhosis or cholecystitis, gall bladder cancer, mesenteric tumor, and costal chondrosarcoma (2).
Geographic origin of the patients is a crucial diagnostic clue for E. vogeli echinococcosis (2). They are typically born in or have lived for prolonged periods in rural tropical areas of Central or South America, particularly in regions with abundant wildlife (2). Familiarity with pacas and whether domestic dogs were fed viscera of pacas are characteristics that contribute to a correct diagnosis (2). Ultrasound fine-needle aspiration and histopathologic examination of surgical specimens can permit taxonomic identification through larval morphologic clues (protoscoleces, hook shape and size, proportions of small and long blades) and are considered the standard, but definitive diagnosis is difficult when the hooks are absent (1–3). Since 2017, in Brazil, using molecular characterization of E. vogeli through cox1 mitochondrial gene in samples from humans, domestic dogs, and pacas has suggested the presence of shared haplotypes among different populations of this cestode, reflecting the retention of ancestral polymorphisms (8–10).
In summary, our report highlights the value of molecular characterization of E. vogeli from histopathologic samples. Consistent with previous reports (2), we recommend that NPE be considered not as a medical curiosity but as a possible diagnosis of polycystic masses in humans from tropical zones in Central or South America.
Dr. Morcillo Muñoz is an infectious diseases fellow at Universidad Nacional de Colombia in Bogotá, Colombia. His research interests primarily focus on neglected infectious diseases.
Acknowledgment
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Wen H, Vuitton L, Tuxun T, Li J, Vuitton DA, Zhang W, et al. Echinococcosis: Advances in the 21st Century. Clin Microbiol Rev. 2019;32:e00075–18. DOIPubMedGoogle Scholar
- D’Alessandro A, Rausch RL. New aspects of neotropical polycystic (Echinococcus vogeli) and unicystic (Echinococcus oligarthrus) echinococcosis. Clin Microbiol Rev. 2008;21:380–401. DOIPubMedGoogle Scholar
- Tappe D, Stich A, Frosch M. Emergence of polycystic neotropical echinococcosis. Emerg Infect Dis. 2008;14:292–7. DOIPubMedGoogle Scholar
- D’Alessandro A, Rausch RL, Morales GA, Collet S, Angel D. Echinococcus infections in Colombian animals. Am J Trop Med Hyg. 1981;30:1263–76. DOIPubMedGoogle Scholar
- D’Alessandro A, Rausch RL, Cuello C, Aristizabal N. Echinococcus vogeli in man, with a review of polycystic hydatid disease in Colombia and neighboring countries. Am J Trop Med Hyg. 1979;28:303–17. DOIPubMedGoogle Scholar
- Botero D, Restrepo MI, Restrepo A. Report of four cases of neotropical polycystic equinococcosis caused by Echinococcus vogeli in Colombia. Curr Trop Med Rep. 2016;3:173–5. DOIGoogle Scholar
- Mondragon-Cardona A, Duran-Gutierrez LF, Yucuma-Gutierrez S, Bolaños F, Vizcaychipi K, Alzate-Carvajal V, et al. Neotropical echinococcosis caused by Echinococcus vogeli in rural Huila, Colombia: report of two last cases in the last 35 years. Int J Infect Dis. 2018;73:306–7. DOIGoogle Scholar
- das Neves LB. Teixeira PEF, Silva S, de Oliveira FB, Garcia DD, de Almeida FB, et al. First molecular identification of Echinococcus vogeli and Echinococcus granulosus (sensu stricto) G1 revealed in feces of domestic dogs (Canis familiaris) from Acre, Brazil. Parasit Vectors. 2017;10:28. DOIGoogle Scholar
- Bittencourt-Oliveira F, Teixeira P, Alencar A, Menezes R, Corrêa C, Neves L, et al. First parasitological, histopathological and molecular characterization of Echinococcus vogeli Rausch and Bernstein, 1972 from Cuniculus paca Linnaeus, 1766 in the Cerrado biome (Mato Grosso do Sul, Brazil). Vet Parasitol. 2018;250:35–9. DOIPubMedGoogle Scholar
- Daipert-Garcia D, Pavan MG, Neves LBD, Almeida FB, Siqueira NG, Santos GBD, et al. Genetic diversity of Echinococcus vogeli in the western Brazilian Amazon. Mem Inst Oswaldo Cruz. 2019;114:
e190149 . DOIPubMedGoogle Scholar
Figures
Cite This ArticleOriginal Publication Date: July 16, 2025
Table of Contents – Volume 31, Number 8—August 2025
| EID Search Options |
|---|
|
|
|
|
|
|
Please use the form below to submit correspondence to the authors or contact them at the following address:
Álvaro A. Faccini-Martínez, Servicio de Infectología, Hospital Militar Central, Tv. 3C No. 49–02, Bogotá, Colombia
Top