Volume 10, Number 7—July 2004
Research
Detection of SARS-associated Coronavirus in Throat Wash and Saliva in Early Diagnosis
Cite This Article
Citation for Media
Abstract
The severe acute respiratory syndrome–associated coronavirus (SARS-CoV) is thought to be transmitted primarily through dispersal of droplets, but little is known about the load of SARS-CoV in oral droplets. We examined oral specimens, including throat wash and saliva, and found large amounts of SARS-CoV RNA in both throat wash (9.58 x 102 to 5.93 x 106 copies/mL) and saliva (7.08 x 103 to 6.38 x 108 copies/mL) from all specimens of 17 consecutive probable SARS case-patients, supporting the possibility of transmission through oral droplets. Immunofluorescence study showed replication of SARS-CoV in the cells derived from throat wash, demonstrating the possibility of developing a convenient antigen detection assay. This finding, with the high detection rate a median of 4 days after disease onset and before the development of lung lesions in four cases, suggests that throat wash and saliva should be included in sample collection guidelines for SARS diagnosis.
Severe acute respiratory syndrome (SARS) is an emerging infectious disease that spread rapidly from China to >30 countries, including Canada, Singapore, Vietnam, and Taiwan, in the first half of 2003 (1–5). In the latest update from the World Health Organization, the number of probable SARS cases is 8,096 (5). The etiologic agent of SARS has been identified as the novel SARS-associated coronavirus (SARS-CoV) (6–9). The disease is highly contagious and has the potential to cause a very large epidemic in the absence of control measures (10,11). Transmission appears to occur primarily through dispersal of droplets from the respiratory tract (12), generated when the patient talks, coughs, or sneezes (4,5,10–13). Although large amounts of SARS-CoV have been reported in sputum and nasal specimens, which may account for transmission during coughing and sneezing (7,14), little is known about the load of SARS-CoV in the oral cavity and how the virus is transmitted during talking. Since sneezing and rhinorrhea are not common symptoms of SARS, and cough with sputum is only seen in the later stage of infection (1–3,13), oral droplets generated during talking may represent an important route of transmission.
We examined specimens derived from the oropharynx and oral cavity, including throat wash and saliva, from 17 patients with probable SARS (15,16). Using a quantitative real-time reverse transcription–polymerase chain reaction (RT-PCR) assay and fractionation experiment, we investigated the load of SARS-CoV in these samples and different components of the throat wash.
Patients
From April 16, 2003, through May 1, 2003, during a 2-week period of the SARS outbreak in Taipei (16), 17 adult patients, who were admitted to the emergency department of the National Taiwan University Hospital and met the clinical case definitions for probable SARS (15), were included in this study. Physicians in the SARS Research Group of National Taiwan University Hospital made the diagnosis for each patient after thorough evaluation of their travel or contact history; symptoms; laboratory data including lymphopenia, thrombocytopenia, and elevated levels of lactate dehydrogenase or creatine kinase; and pneumonic patch in the chest x-ray. The first day of fever was defined as day 1 of illness. The serologic test of an indirect immunofluorescence assay performed on serum specimens collected 28 days after onset confirmed SARS-CoV infection in 13 of the 17 patients. The other four patients had at least two positive real-time RT-PCR results. Therefore, all 17 cases with probable SARS in this study were confirmed by laboratory tests (15).
Sample Processing
With the patient’s consent, saliva and throat wash (by gargling 10 mL normal saline) were collected in an airborne isolation room, according to the guidelines for aerosol-generating procedures (17). All samples were transferred to the biosafety level 3 (BSL3) laboratory and stored at –80°C until use (18). After thawing, 5 mL of the throat wash was centrifuged at 1,500 x g for 15 min to separate the supernatant from the mucous-cell pellet. Four microliters of the supernatant were collected as the throat wash supernatant. The remaining 1-mL portion that contained the mucous-cell pellet was treated with equal volume of N-acetyl-L-cysteine at room temperature for 25 min and centrifuged at 1,500 x g for 15 min to further separate the cell pellet from the supernatant, of which 1.12 mL was collected as the treated supernatant of throat wash. Instead of extensively washing the potentially contagious cell pellet, we kept the remaining 0.88 mL as the cellular fraction of throat wash. Equal amounts of the supernatant, treated supernatant, and cellular fractions were subjected to viral RNA extraction. An aliquot of the saliva, to which an equal volume of 1 x phosphate-buffered saline (PBS) was added, was also subjected to viral RNA extraction.
Isolation of Viral RNA
Viral RNA was isolated from aliquots of saliva and different fractions of throat wash from the 17 probable SARS patients and 12 healthy controls by using the QIAamp viral RNA mini kit (Qiagen, Hilden, Germany) in the BSL3 laboratory (18). Viral RNA was also isolated from culture supernatants of the SARS-CoV isolate, TW1 (19), human coronavirus 229E strain, and human enteric coronavirus Dallas 1 strain (American Type Culture Collection, Manassas, VA).
Quantitative Real-Time RT-PCR
The assay used forward and reverse primers and a fluorogenic probe of the SAR1S_AS Taqman assay design (Applied Biosystems, Foster City, CA). They matched to a region within a previously described region of the ORF1b (6,7), which is also completely conserved by different isolates of SARS-CoV (Figure 1A) (20,21). The sequences of the forward primer, reverse primers, and probe are 5′-CACACCGTTTCTACAGGTTAGCT-3′ (genome positions 15316 to 15338 of the Urbani strain) (20), 5′-GCCACACATGACCATCTCACTTAAT-3′ (positions 15380 to 15356) and 5′-ACGGTTGCGCACACTCGGT-3′ (positions 15355 to 15339), respectively. A 200-bp product covering this region was generated by using the primers (F1 and R1), the Superscript II one-step RT-PCR system (Invitrogen, San Diego, CA), and the RNA template derived from the SARS-CoV TW1 strain (19). The sequences of the primers F1 and R1 are 5′-CAGAGCCATGCCTAACATGC-3′ (genome positions 15239 to 15258) (20) and 5′-GCATAAGCAGTTGTAGCATC-3′ (positions 15439 to 15420), respectively. RT-PCR conditions were 52°C for 40 min and 94°C for 2 min, followed by 35 cycles of 94°C for 1 min, 60°C for 1 min, and 68°C for 45 s. The product was subsequently cloned into the TA cloning vector (Invitrogen, San Diego, CA) to generate the construct, ORF1b/pCRII-TOPO (Figure 1B). The in vitro transcribed RNA was purified and quantified to determine the copy number of RNA as described previously (22). An aliquot (5 μL) of RNA isolated from the clinical sample and known amounts of the in vitro transcribed RNA (5 to 50 million copies) were subjected to real-time RT-PCR by using the SAR1S_AS primers, probe, and the Taqman one-step real-time RT-PCR master mix reagent kit (Applied Biosystems). The amplification conditions were 48°C for 30 min and 95°C for 10 min, followed by 45 cycles of 95°C for 15 s and 60°C for 1 min. The ABI prism 7000 sequence detector was used to analyze the emitted fluorescence during amplification. A positive result is defined by the cycle number (CT value) required to reach the threshold as described previously (22). Precautions for PCR were followed to avoid contamination (23). Since 5 μL of 50 μL RNA eluates that were derived from 560 μL throat wash supernatant, was used in each reaction, the number of SARS-CoV RNA copies per reaction was divided by 56 μL (560 μL x 5 μL/50 μL) and multiplied by 1,000 to determine the RNA copies per milliliter. The sensitivity of the assay is 5 copies RNA per reaction, corresponding to 90 copies per milliliter throat wash.
SARS-CoV RNA in Components of Throat Wash
We used the following formula to calculate the copy numbers of SARS-CoV RNA in different components including the supernatant (S), the mucus-associated (M), and the cell-associated (C) components in the 5-mL throat wash, which was the starting volume in our fractionation experiment. The numbers of RNA copies in the S component equal the amount (copies/mL) in the supernatant times 5 (mL) (S = supernatant x 5 mL). Since treatment of the mucus-cell pellet with N-acetyl-L-cysteine presumably released SARS-CoV from the mucus and increased the volume twofold (from 1 mL to 2 mL), the copy numbers in the M component equal the amount in the treated supernatant (copies/mL) times 2 mL minus that from the originally untreated supernatant (1 mL) (M = treated supernatant x 2 mL – supernatant x 1mL). The copy numbers in the C component equal the amount in the cellular fraction (copies/mL) times the volume of the cell pellet (C = cellular fraction x volume of cell pellet [in mL]). Taking patient ID17 as an example, S = 4,790 copies (958 x 5), M = 8,402 copies (4,680 x 2 – 958 x 1), and C = 330 copies (8,460 x 0.039). The amount of SARS-CoV RNA in the cell-free component, which equals the amount in the S component plus that in the M component, is 13,192 copies, corresponding to 97.5% of the total SARS-CoV RNA in 5 mL throat wash, and that in the cell-associated component is 330 copies, corresponding to 2.5% of the total (Table 1).
Immunofluorescence Assay
Aliquots of the cellular fraction of throat wash from patients and six healthy controls were fixed onto 12-well Teflon-coated slides and subjected to a previously described immunofluorescence assay (19). The first antibody was serum from a rabbit immunized with the recombinant nucleocapsid protein of the SARS-CoV (prepared by P. J. Chen), and the secondary antibody was the FITC-conjugated goat anti-rabbit immunoglobulin G (Pierce Biotechnology, Rockford, IL).
Statistical Analysis
Regression analysis was performed to examine the correlation between the sampling day and the amount of SARS-CoV RNA in throat wash or saliva and the correlation between the amount in throat wash and saliva (software SPSS base 10.0, SPSS Inc., Chicago, IL).
The demographic and clinical information of the 17 patients are summarized in Table 2. Viral RNA was extracted from saliva and supernatant of the throat wash and then subjected to a quantitative real-time RT-PCR assay by using the primers and probe within a highly conserved region of the ORF1b (Figure 1A) (20,21). Known amounts of the in vitro transcribed RNA covering this region were used as the standard for quantification. As shown in Figure 1B, a linear curve was observed as the input RNA increased from 5 to 50 million copies per reaction. Positive signal was detected in the reactions containing RNA template derived from the SARS-CoV Taiwanese strain TW1 but not in those from 12 healthy controls and from two human coronaviruses (229E strain and human enteric coronavirus Dallas 1 strain) and not in the reaction containing no RNA (data not shown) (19,22).
The results of the real-time RT-PCR assay on the throat wash and saliva are summarized in Table 2. The sampling day of these patients varied from day 2 to day 9 after onset of fever, with a median of day 4. SARS-CoV RNA was readily detected in throat wash from all 17 patients. The amount of the SARS-CoV RNA in the throat wash was 9.58 x 102 to 5.93 x 106 copies per mL (median 3.56 x 103 copies/mL). SARS-CoV RNA was also detected in saliva from all 14 available specimens. The amount of SARS-CoV RNA in the saliva was 7.08 x 103 to 6.38 x 108 copies per mL (median 9.92 x 104 copies/mL). The amount of SARS-CoV RNA in the throat wash or saliva does not correlate with the sampling day (simple linear regression, coefficient of correlation r = 0.106 and 0.147, respectively), underscoring a more complex course of virus-host interaction. The amount of SARS-CoV RNA in the saliva was greater than that in the throat wash for every patient from whom both type of specimens were available. A linear relationship existed between the amounts of SARS-CoV in the saliva and throat wash (simple linear regression, r = 0.848, p < 0.005), which suggests, but does not prove, that they could originate from a common source in the respiratory tract.
To further investigate whether SARS-CoV is also present in the cellular component of the throat wash, we carried out the fractionation experiment and examined the amount of SARS-CoV RNA in different components. As shown in Table 1, SARS-CoV RNA was detected in the cell-associated component of the throat wash from all 16 specimens examined. The range of viral load was 34–4222,000 copies per 5 mL throat wash. While 0.1%–11.2% of SARS-CoV RNA in the throat wash is present in the cell-associated component, a greater proportion of SARS-CoV RNA, 88.8% to 99.9%, is present in the cell-free component. This finding suggests that SARS-CoV is released very efficiently. The possibility that virus is released from the cells during thawing is unlikely, since the fractionation experiment performed for aliquots of some samples without prior freezing and thawing showed a similar result. For example, in ID7 the percentages of the cell-associated and cell-free components for an aliquot performed without freezing were 99.87% and 0.13%, respectively. These are similar to the results of 99.9% and 0.1% for another aliquot performed after freezing and thawing (Table 1).
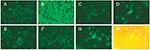
Electron microscopic studies have shown SARS-CoV particles in the desquamated cells from bronchoalveolar lavage and lung tissues, both in the lower respiratory tract (6,8,24). Our detection of SARS-CoV RNA in the cell-associated component of the throat wash suggested that SARS-CoV also replicates in the upper respiratory tract. To further explore this possibility, we prepared spot slides from the cellular fraction of the throat wash and examined them with an indirect immunofluorescence assay by using a polyclonal serum from a rabbit immunized with a recombinant nucleocapsid protein of SARS-CoV. When used in the epithelial cells prepared from two randomly chosen specimens (ID11 and ID17), the postimmune serum, but not the preimmune serum, reacted with epithelial cells with a speckle pattern (Figure 2, C–D and F–G). The classification of these cells as epithelial cells was supported by the size and morphologic features of them under light microscope (Figure 2H). Only background signal was seen in the cells prepared from a healthy control (Figure 2E). These findings indicate that SARS-CoV can replicate in the epithelial cells of the upper respiratory tract, and such cells can be used in an antigen detection assay.
We report large amounts of SARS-CoV RNA in the throat wash and saliva from probable SARS case-patients. This finding supports the possibility that SARS-CoV can be transmitted through oral droplets. Most coronaviruses are known to replicate in the epithelial cells of the respiratory or enteric tract. After budding into the pre-Golgi compartment, virus particles are released through an exocytosis-like process at the apical or basolateral surface, or both (25). Apical release is likely to facilitate the spread of virus in the respiratory or enteric tract, whereas basolateral release facilitates systemic spread. Our findings that SARS-CoV can be detected in cells derived from throat wash by the immunofluorescence assay and that most of the SARS-CoV in throat wash is present in the cell-free component suggest that after its replication in the epithelial cells, SARS-CoV is released efficiently and accumulates in the oropharynx and oral cavity, which may contribute to its transmission through oral droplets.
Practicing droplet and contact precautions prevents nosocomial transmission of SARS among healthcare workers (26). However, a cluster of SARS cases was reported among apparently protected healthcare workers during aerosol-generating procedures performed on SARS patients (27). This finding led to the controversial hypothesis of airborne transmission of SARS, in which very small particles (<5 μm) are spread in the air (17,27). A substantial proportion, 88.8% to 99.9%, of the SARS-CoV in the throat wash was present in cell-free form; this finding offers a mechanistic explanation of the possibility of airborne transmission of SARS.
Three types of specimens from the upper respiratory tract, nasopharyngeal aspirates, nasopharyngeal swab, and oropharyngeal swab have been recommended to detect SARS-CoV (28,29). However, RT-PCR performed on nasopharyngeal aspirates from SARS patients had positive rates of 32% at day 3, 50% at day 5, and 68% at day 14 (8,14). A recent study using nasopharyngeal aspirates reported a positive rate of 71% at a mean of 4.4 days (30). In this study, we reported that SARS-CoV RNA can be detected in both throat wash and saliva from all specimens examined at an average sampling day 4.8 (range day 2–9). Furthermore, specimens of throat wash from four of our study participants who came to our emergency department, a designated SARS screening site in Taipei during the SARS outbreak, were collected when radiographic evidence of pneumonia or respiratory distress syndrome had not been observed (Table 2). To our knowledge, this report is the first showing that SARS-CoV could be detected in probable SARS patients before lung lesions developed.
The high SARS-CoV detection rate in our study contrasts with those reported previously by using nasopharyngeal aspirates (8,14,30). One possibility is that more SARS-CoV are present in the oropharynx and oral cavity than in the nasopharynx. In spite of the differences in the dilutional factors (for example, 10 mL of normal saline in the throat wash, 1.5–2 mL in the nasopharyngeal aspirates, and none in the saliva), the amounts of SARS-CoV RNA in the throat wash and saliva in our study, 9.58 x 102 to 6.38 x 108 copies per mL, were in the same range as those previously reported for the nasopharyngeal specimens (103–108 copies/mL) (7,14). A mutually nonexclusive possibility is that more respiratory secretions, mucus, and cells can be removed from the respiratory tract through throat wash than through nasopharyngeal aspiration, nasopharyngeal swabs, and oropharyngeal swabs. Regardless of the reason, SARS-CoV was also detected in the throat wash of nine SARS patients in a previous report (6), which suggests the need to evaluate the benefit of collecting throat wash to diagnose SARS.
To our knowledge, this report is the first that demonstrates the possibility of devising a SARS-CoV antigen detection assay by using cells derived from throat wash. Since a small number of patients were examined, future study with more patients and controls is required to develop a useful diagnostic test. Technically, throat wash and saliva are easier to collect when compared with the collection of currently recommended respiratory specimens (28,29). In addition, they can be obtained without close contact between the patient and healthcare worker, and thus reduce the risk for infection of healthcare workers. Another commonly obtained sample is sputum; however, it is rarely available at the early stage of infection, when virtually no cough or only dry cough is present (1–3,13). These features, together with the high detection rate at early stage and before the development of lung lesions, suggest that throat wash and saliva are ideal specimens for early diagnosis of SARS and should be included in guidelines for sample collection for SARS diagnosis (28,29). Further studies with longitudinally collected throat wash and saliva specimens from a larger number of SARS patients would help determine the onset and duration of infectiveness, extent of infectiveness of some patients, such as superspreaders, and the response to antiviral agents.
Dr. Wei-Kung Wang is an associate professor of virology at the Institute of Microbiology, College of Medicine, National Taiwan University. His research interests include study of viral load, immune activation markers, and the pathogenesis of viral infectious diseases, such as dengue virus and SARS-CoV.
Acknowledgments
We are indebted to all the medical personnel at the National Taiwan University Hospital for taking care of SARS patients during the outbreak in Taipei and members of the SARS Research Group of the National Taiwan University College of Medicine/National Taiwan University Hospital, including Ding-Shinn Chen, Yuan-Teh Lee, Hong-Nerng Ho, Chu-Min Teng, Ming-Fu Chang, Bor-Liang Chiang for discussion and coordination, Tun-Hou Lee for critical comments, and Chao-Min Lee for assistance.
Applied Biosystems Taiwan Inc. provided an ABI prism 7000 sequence detector during the outbreak. This work was supported by the National Science Council (NSC92-2751-B-002-006-Y), Taiwan.
References
- Tsang KW, Ho PL, Ooi GC, Yee WK, Wang T, Chan-Yeung M, A cluster of cases of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1977–85. DOIPubMedGoogle Scholar
- Lee N, Hui D, Wu A, Chan P, Cameron P, Joynt GM, A major outbreak of severe acute respiratory syndrome in Hong Kong. N Engl J Med. 2003;348:1986–94. DOIPubMedGoogle Scholar
- Poutanen SM, Low DE, Henry B, Finkelstein S, Rose D, Green K, Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348:1995–2005. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Update: outbreak of severe acute respiratory syndrome—worldwide. MMWR Morb Mortal Wkly Rep. 2003;52:241–8.PubMedGoogle Scholar
- World Health Organization. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. [2003 May 14]. Available from: http://www.who.int/csr/sars/country/2003_04_21/en/
- Ksiazek TG, Erdman D, Goldsmith C, Zaki SR, Peret T, Emery S, A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–66. DOIPubMedGoogle Scholar
- Drosten C, Gunther S, Preiser W, Werf S, Brodt HR, Becker S, Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–76. DOIPubMedGoogle Scholar
- Peiris JSM, Lai ST, Poon LLM, Guan Y, Yam LYC, Lim W, Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–25. DOIPubMedGoogle Scholar
- Kuiken T, Fouchier RAM, Schutten M, Rimmelzwann GF, Amerongen G, Riel D, Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–70. DOIPubMedGoogle Scholar
- Riley S, Fraser C, Donnelly CA, Ghani AC, Abu-Raddad LJ, Hedley AJ, Transmission dynamics of the etiological agent of SARS in Hong Kong: impact of public health interventions. Science. 2003;300:1961–6. DOIPubMedGoogle Scholar
- Lipsitch M, Cohen T, Cooper B, Robins JM, Ma S, James L, Transmission dynamics and control of the severe acute respiratory syndrome. Science. 2003;300:1966–70. DOIPubMedGoogle Scholar
- World Health Organization. Consensus document on the epidemiology of severe acute respiratory syndrome (SARS). [cited 2004 Feb 18]. Available from: http://www.who.int /csr/sars/en/index.html
- Donnelly CA, Ghani AC, Leung GM, Hedley AJ, Fraser C, Riley S, Epidemiological determinants of spread of causal agent of severe acute respiratory syndrome in Hong Kong. Lancet. 2003;361:1761–6. DOIPubMedGoogle Scholar
- Peiris JSM, Chu CH, Cheng VCC, Chan KS, Hung IFN, Poon LLM, Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1773–8. DOIPubMedGoogle Scholar
- World Health Organization. Case definitions for surveillance of SARS. [cited 2003 May 1]. Available from: http://www.who.int/csr/sars/casedefinition/en/
- Centers for Disease Control and Prevention. Severe acute respiratory syndrome—Taiwan 2003. MMWR Morb Mortal Wkly Rep. 2003;52:461–6.PubMedGoogle Scholar
- Centers for Disease Control and Prevention. Interim domestic infection control precautions for aerosol-generating procedures on patients with severe acute respiratory syndrome (SARS). [cited 2003 Oct 4]. Available from: http://www.cdc.gov/ncidod/sars/aerosolin fectioncontrol.htm.
- Centers for Disease Control and Prevention. Interim laboratory biosafety guidelines for handling and processing specimens associated with severe acute respiratory syndrome (SARS). [2003 Oct 4]. Available from: http://www.cdc.gov/ncidod/sars/sarslabguide.htm
- Hsueh PR, Hsiao SH, Yeh SH, Wang WK, Chen PJ, Wang JT, Microbiologic characterization, serologic responses, and clinical manifestations in severe acute respiratory syndrome, Taiwan. Emerg Infect Dis. 2003;9:1163–7.PubMedGoogle Scholar
- Rota PA, Oberste MS, Monroe S, Allan NW, Campagnoli R, Icenogle JP, Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–9. DOIPubMedGoogle Scholar
- Marra MA, Jones SJM, Astell CR, Holt RA, Brook-Wilson A, Butterfield YSN, The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–404. DOIPubMedGoogle Scholar
- Wang WK, Sung TL, Tsai YC, Kao CL, Chang SM, King CC. Detection of dengue virus replication in peripheral blood mononuclear cells from dengue virus type 2-infected patients by a reverse transcription-real-time PCR assay. J Clin Microbiol. 2002;40:4472–8. DOIPubMedGoogle Scholar
- Kwok S, Higuchi R. Avoiding false positive with PCR. Nature. 1989;339:237–8. DOIPubMedGoogle Scholar
- Nicholls JM, Poon LLM, Lee KC, Ng WF, Lai ST, Leung CY, Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773–8. DOIPubMedGoogle Scholar
- Lai MMC, Holmes KV. Coronaviridae: the virus and their replication. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, et al., editors. Fields virology, 4th ed. Philadelphia: Lippincott William and Wilkins; 2001. p. 1163–85.
- Seto WH, Tsang D, Yung RWH, Ching TY, Ng TK, Ho M, Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS). Lancet. 2003;361:1519–20. DOIPubMedGoogle Scholar
- Centers for Disease Control and Prevention. Cluster of severe acute respiratory syndrome cases among protected health-care workers—Toronto, Canada, April 2003. MMWR Morb Mortal Wkly Rep. 2003;52:433–6.PubMedGoogle Scholar
- World Health Organization. Sampling for severe acute respiratory syndrome (SARS) diagnostic tests. [2003 Oct 4]. Available from: http://www.who.int/csr/sars/sampling/en/
- Centers for Disease Control and Prevention. Guidelines for collection of specimens from potential cases of SARS. [cited 2003 Dec 26]. Available from: http://www.cdc.gov /ncidod/sars/ specimen_collection_sars2.htm
- Tsang OTY, Chau TN, Choi KW, Tso EYK, Lim W, Chiu MC, Coronavirus-positive nasopharyngeal aspirate as predictor for severe acute respiratory syndrome mortality. Emerg Infect Dis. 2003;9:1381–7.PubMedGoogle Scholar
Figures
Tables
Cite This ArticleTable of Contents – Volume 10, Number 7—July 2004
| EID Search Options |
|---|
|
|
|
|
|
|

Please use the form below to submit correspondence to the authors or contact them at the following address:
Shan-Chwen Chang, Department of Internal Medicine, National Taiwan University Hospital, No. 7 Chung-Shan South Road, Taipei 100, Taiwan; fax: 8862-23971412
Top